Favism is an inherited deficiency of the enzyme G6PD, which produces the important antioxidant NADPH. The G6PD gene is located on the x-chromosome at Xq28.
There is a joke among biochemists that Pythagoras (of right triangle fame) would never eat a falafel. This inside joke revolves less around the falafel, considering there was probably no such dish in Ancient Greece, and more on the contents of the falafel. Fava beans, the core ingredient in falafel, is what Pythagoras had a problem with. He famously advised his students “…Be far from the fava bean consumption…”
Fava beans and the biochemists joke is related to a disease called Favism (after the Fava bean, which we’ll get to later).
Recommended Video for you:
What Is Favism?
Favism is an inherited disorder that causes a deficiency of an enzyme called Glucose-6-phosphate dehydrogenase, or G6PD for short. Deficiency of this enzyme causes anemia in its patients.
The gene for this enzyme is located on the X chromosome of the sex chromosome pair, meaning that a mutation in the G6DP gene in an XY combination leads to a deficiency of the enzyme. Females with XX combinations would require both copies of the X chromosomes to have a mutation in the gene.
The gene is located at position 28 on the long arm of the X chromosome, and is denoted as Xq28.
There are 2 normal versions of the G6PD gene – GdA+ and GdB. There can be hundreds of different disease-causing mutations that can be categorized roughly depending on the origin – GdA- of African origin and GdMed of Mediterranean origin.
Not all individuals with mutations in this gene will have a deficiency to the same extent. Some individuals only have mild hemolytic anaemia, which can be managed and treated, while in other individuals the response to certain drugs and foods can be life-threatening!
What Is The G6PD And What Does It Do In The Body?
G6PD is an enzyme that catalyses the first reaction in the pentose phosphate pathway. The pentose phosphate pathway is an elaborate series of reactions meant to create more of a molecule called NADPH.
Cells use NADPH when they are building new biomolecules or as a protective measure against oxidative stress.
Oxidative stress occurs when a cell has a build-up of certain free radicals. These free radicals are highly reactive molecules or ions produced as byproducts during metabolic reactions. Free radicals like peroxide, hydroxide radical (OH–), superoxides, singlet oxygen (oxygen with an unpaired electron), and alpha-oxygen are called Reactive Oxygen Species or ROS. These ROS can react with biomolecules like proteins, lipids and nucleic acids (DNA and RNA), causing them to become defective. This can eventually lead to mutation or cell death.
How Does A Deficieny Of G6PD Cause Favism?
In most cells, a G6PD deficiency is fine, as they have other ways of protecting themselves from oxidative stress. Most cells in the body make certain protective proteins in times of cellular stress.
However, this is not so for your Red Blood Cells (RBCs)
RBCs do not have a nucleus or genetic material, so they cannot make any new proteins, protective or otherwise. They, therefore, rely solely on certain built-in protective mechanisms. NADPH produced by G6PD plays a central role in this protection.
Since G6PD is the first enzyme in the pentose phosphate pathway, its deficiency is a bottleneck to the rest of the pathway. If the rest of the reactions in the pathway cannot happen fast enough, then there won’t be enough NADPH in the cells which causes a problems.
How Does NADPH Protect The Cell From Oxidative Stress?
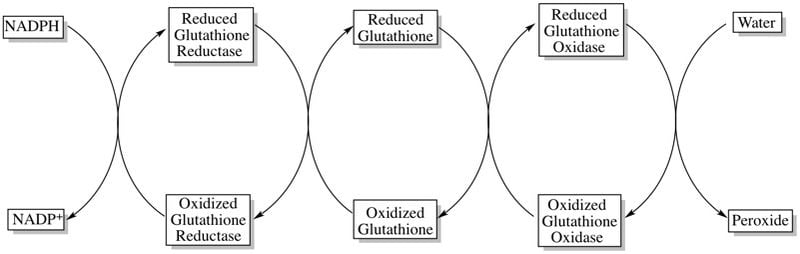
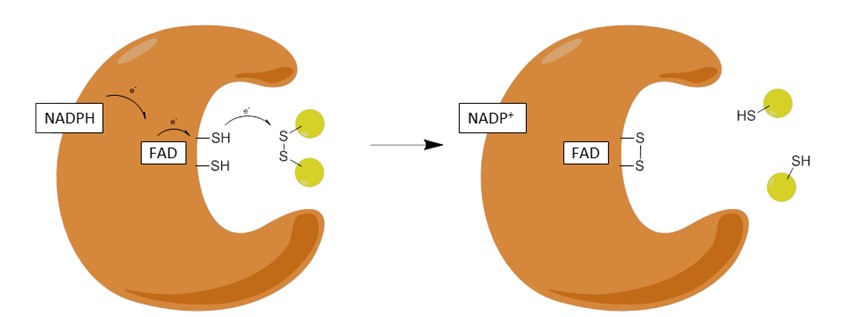
NADPH interacts with another protein called glutathione to shield the cell from free radicals.
Glutathione exists in two forms—a reduced form (GSH) and an oxidized form (GSSG). The first step of the antioxidant defense is NADPH reducing GSSG to GSH.
These reduced glutathione molecules go on to reduce free radicals, especially peroxides, which are the biggest threats to the RBCs. Reducing the free radicals prevents them from wrecking havoc in the cell.
The glutathione returns once more to its oxidized state as GSSG waiting to be reduced by another NADPH molecule. An NADP+ molecule is generated in the first reaction, which goes on to G6PD to get reduced again, repeating the cycle over and over.
Without enough G6PD, the RBC will have a shortage of NADPH. Without NADPH, glutathione won’t be able to reduce free radicals, and the result will be chaos! Anarchist free radicals will oxidize lipids that make up the cell membrane and the proteins that hold the membrane together.
The RBC’s walls get weaker and weaker until it breaks apart, a condition called hemolytic anaemia. The RBCs that burst open will spill their toxic free radicals into their surroundings, where they can cause even more damage to the surrounding cells.
How Do Fava Beans Worsen Favism?
The culprit molecules in the beans are vicine and convicine. These molecules, otherwise inert, get converted into a toxic free radical, divicine, by gut bacteria. The intestines absorb divicine, so it ends up in the blood, where it poses a risk in individuals with favism.
Divicine produces more free radicals in RBCs, which pile up, finally causing hemolysis. If too many RBCs are lost, a person will experience a case of hemolytic anaemia! The anti-malarial drug used in Korean War behaves in a similar way, which explains the mysterious ailment that affected so many soldiers.
Conclusion

Did Pythagoras know about fava bean’s disease-causing capabilities? It’s contentious. His motives against the fava bean might be more superstition than knowledge of the disease itself.
Philosophers like Aristotle and many more throughout the ages have speculated about the reason for Pythagoras’ aversion to this bean. Reasons ranged from superstition, since the beans looked like the gates of Hades, or perhaps because they looked like genitals, testicles or eggs.
Orphics believed that the beans harbored the souls of the dead, and said, “Eating fava beans and gnawing on the heads of one’s parent are one and the same thing”. Some like Diogenes Laertius believed in Pythagoras’ logic, proposing that because the beans caused gas and an upset stomach, they were to be avoided.
What Pythagoras actually believed is anyone’s guess, but it was these very beans that caused his ultimate death. Refusing to pass through a fava bean field, Pythagoras was caught by his enemies and killed in 495 BC. Tragic irony, indeed.
References (click to expand)
- (PDF) Favism – from the “avoid fava beans” of Pythagoras to .... Academia.edu
- Beutler, E. (2008, January 1). Glucose-6-phosphate dehydrogenase deficiency: a historical perspective. Blood. American Society of Hematology.
- Adkison, L. R. (2012). Hematologic Genetics and Disorders. Elsevier's Integrated Review Genetics. Elsevier.













