Everything that has ever existed or ever will exist has been made possible by some permutation or combination of the elements found on a periodic table. That colorful array of elements holds an entire universe of information.
The periodic table makes our lives much easier, but also more difficult, all at the same time! It not only helps us remember and understand our elements, but also gives rise to deep existential questions, such as how did these elements come into existence at all?
Recommended Video for you:
The Appearance Of Matter
This attempt to discover the origins of chemical elements takes us back to the beginning of time.
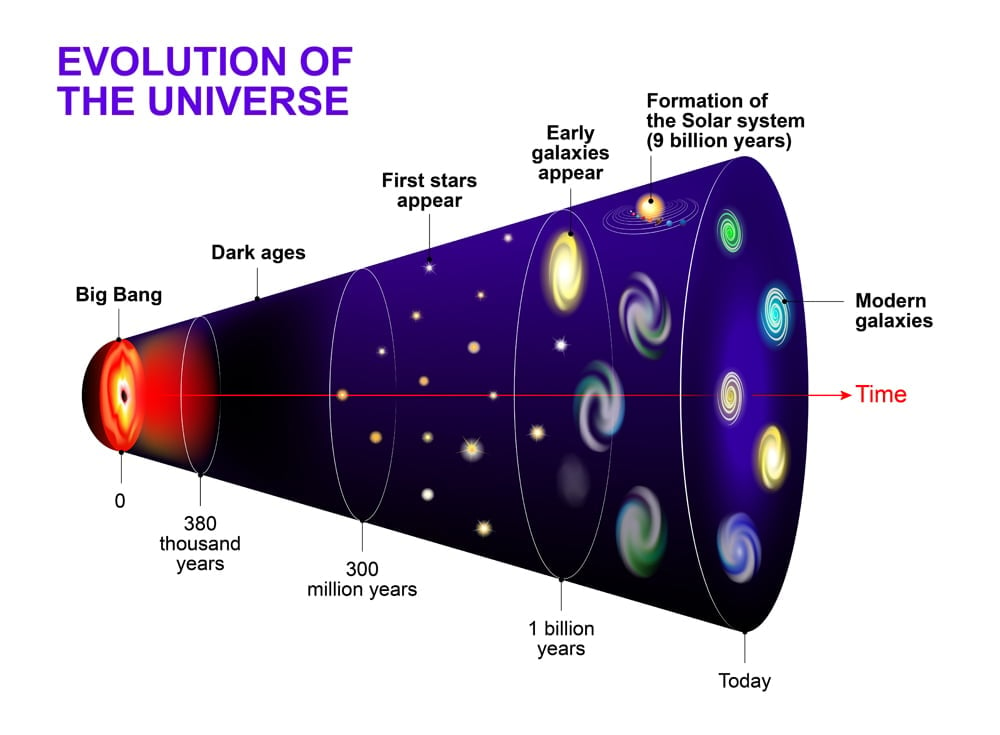
Immediately after the Big Bang, the universe was a dense soup of matter and energy. The temperature was around 1032 Kelvin. The universe started inflating and simultaneously cooling off (though it was still trillions of Kelvin). The elementary particles (quarks and electrons) started popping into existence.
The Appearance Of Protons And Anti-protons
When the universe was slightly less than 0.0001 seconds old, it started experiencing a new form of disturbance. The cosmic energy, which had been hanging around as high-energy radiation, began colliding with each other.
These collisions produce particle (protons) and antiparticles (anti-protons) in a process called pair formation.
The universe continuously churned out more and more of these pairs. On the other hand, these proton and anti-proton pairs were annihilating each other and transforming back into photons and radiation.
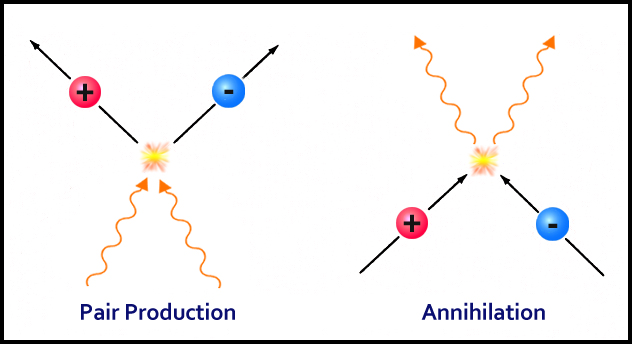
Now, at the age of 0.0001 seconds, the universe was a bit cooler and the photons had stopped forming new pairs, but the already formed opposite pairs continued to annihilate each other.
One would think that eventually, there wouldn’t be any protons, left but as luck would have it, the process of pair production was slightly more inclined towards protons (we still don’t know why). After all the processes ceased, the universe was left as mostly photons, along with light sprinkles of protons.
The Appearance Of Neutrons
The rapidly expanding universe caused some protons to smash against electrons, giving birth to neutrons (for every 7 protons, there is 1 neutron). At this point, the universe was a few seconds older and much colder (only one billion Kelvin).
The protons and neutrons came together to form the nucleus/ion of the first element Hydrogen (H), which further fused with another hydrogen nucleus to form a Helium (He) nucleus. Three minutes after the Big Bang, and the ratio is now 75% H ions and 25% He ions (along with a very negligible amount of Li-ions). The elements are in ionic form because the universe is still very hot—too hot to form atoms.
Approximately 380,000 years after the Big Bang was the epoch of recombination. After years of expanding and cooling the universe was finally ready for the nuclei to capture the electrons. The ions of H and He recombine with electrons and forms the first stable atoms (imagine how easy chemistry class would have been at this point!), giving us our first form of light, and effectively initiating the chemical evolution.
However, after the recombination era, the universe went dark again.
Nucleosynthesis And The Life Of Stars
Over the course of time, the universe further cooled down, dense gas clouds came together due to gravity, and created the first star-forming regions. As the clouds got smashed together, they started forming hot and heavy cores that didn’t want to get any bigger. The hot core started burning itself to prevent even more clouds from clumping together. Thus began a contest between the strength of gravity and the burning pressure in the condensed core. The point at which these two forces come to equilibrium is when a star is born!
Over countless millennia, many galaxies were formed, each with millions of twinkling stars big and small. And what keeps them bright? Their burning cores.
To keep their cores from collapsing under gravity, the stars needed to tap into a constant source of energy. This energy was eagerly provided by the release of binding energy.
Imagine that 4 hydrogen atoms come together in the core of the sum; two protons from its nucleus remain, while the other two turn into neutrons(n) with the help of quantum tunneling.
Once fused, they form a helium nucleus. The helium that is formed weighs slightly less than the total mass of 2n and 2p. The missing mass is what is converted into binding energy and ultimately fuels a star. One such reaction releases 26.71 megaelectron volts of energy… now imagine millions of those interactions occurring at blinding speeds!
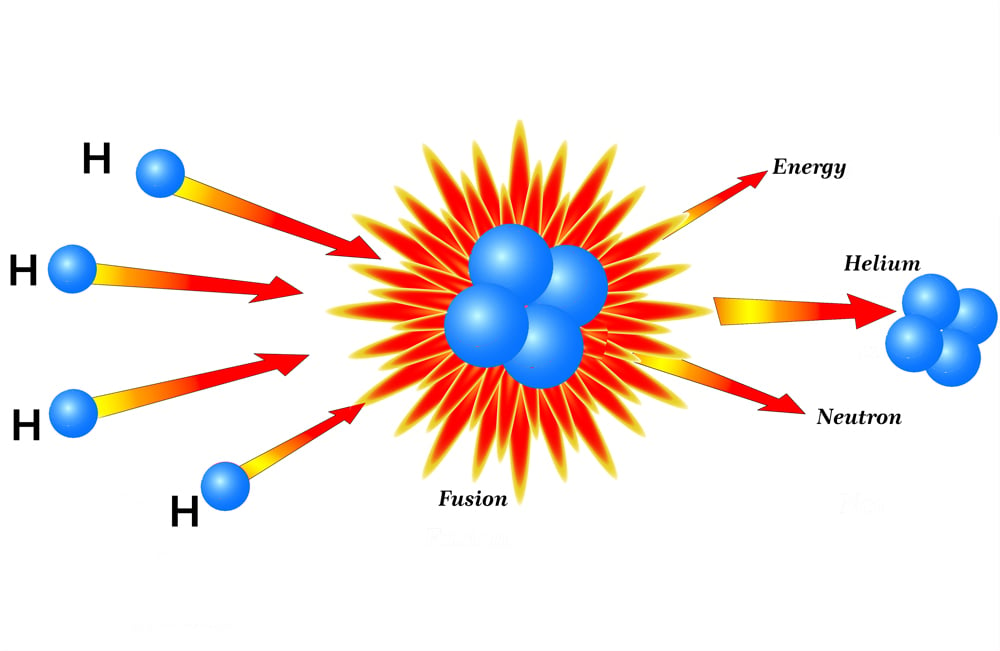
Throughout the life of a star, it undergoes different stages of burning fuel to keep itself from collapsing. This process gives rise to the stellar phenomenon of nucleosynthesis, which begins with hydrogen-burning or fusion. A star spends 90% of its lifetime fusing hydrogen into helium. After the hydrogen has been depleted, it begins fusing helium into higher elements. With each new stage of elemental fusion, the core becomes denser, and the outer layers of the stars begin to expand, gradually turning into a red giant.
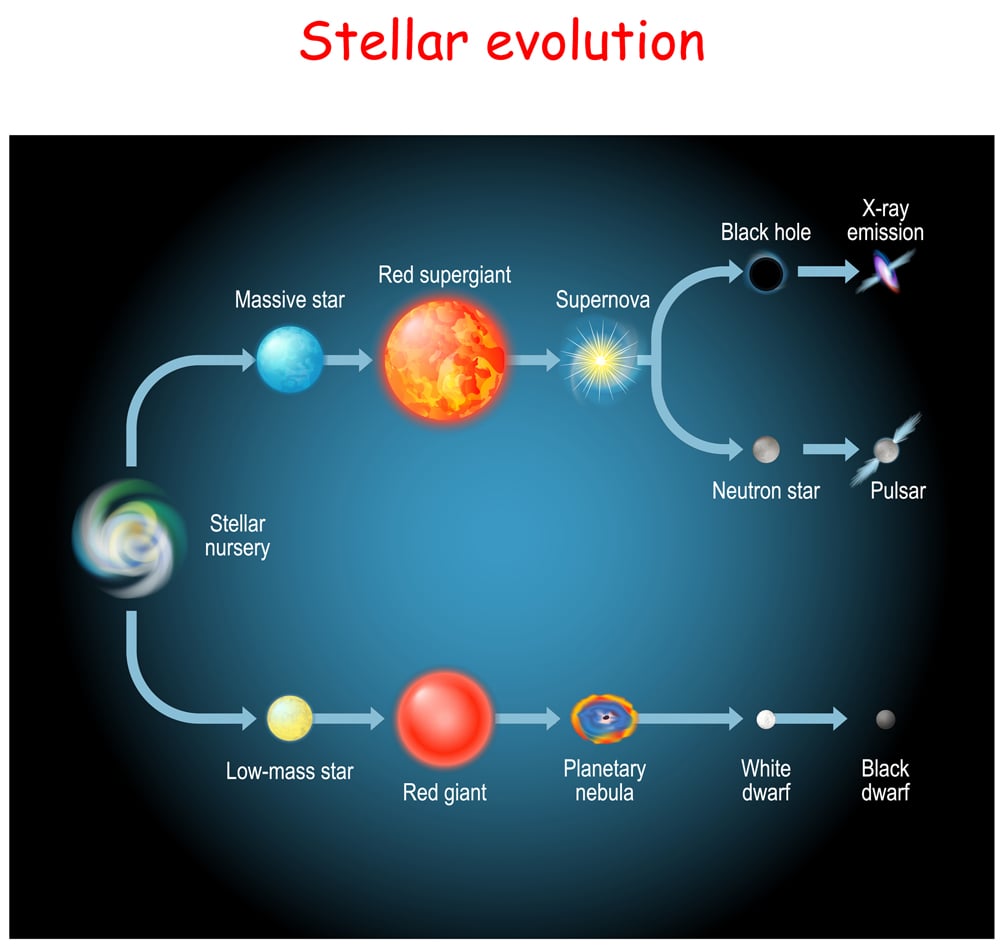
Stars roughly equivalent to the mass of our sun (or lighter) can produce elements above helium only after turning into a red giant (which means it is about to die), as their cores aren’t hot enough. However, the cores of high mass stars make perfect cauldrons for the fusion of nuclei heavier than helium to generate energy. From this point forward in the article, we will only be looking at massive stars.
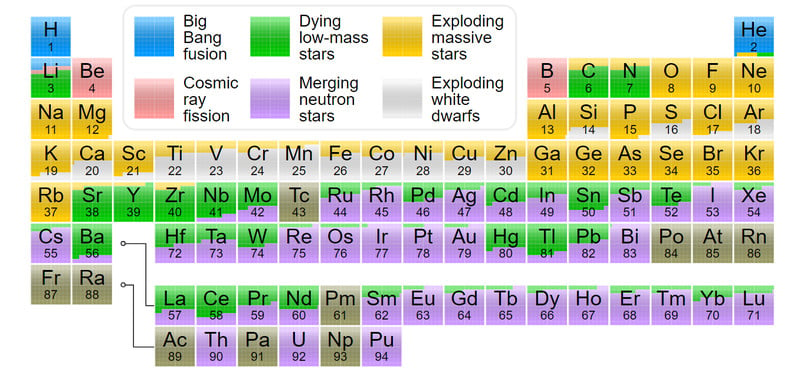
Two Helium atoms fuse to give carbon, which then fuses with another helium to create oxygen, which this goes on to make all the elements on the periodic table up to Silicon, except for Boron and Beryllium, which are not formed in a star, but are instead formed by cosmic fission.
The final lap of stable stellar evolution is when silicon burning begins. Once the core starts fusing silicon to iron, the star’s days are truly numbered. Soon, the core will have no more nuclear reactions to “win” against gravity. Iron has the most stable nucleus in the universe and fusing it to something heavier doesn’t release energy, but actually requires external energy. This marks the beginning of the end of a massive star’s life.
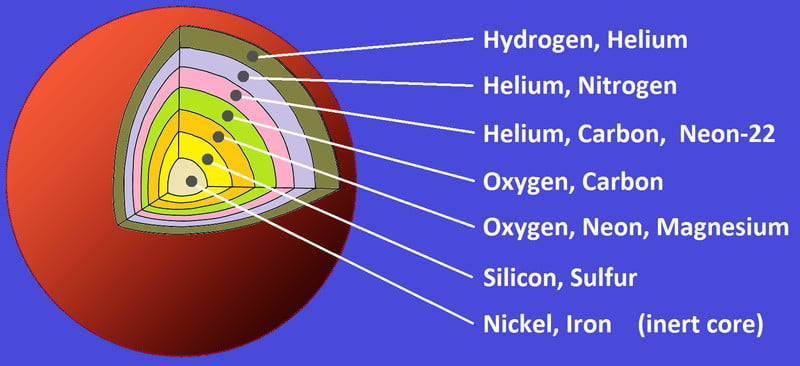
When the core has only iron (and traces of nickel), it becomes so dense that it starts collapsing in on itself. In the last few minutes, the star looks layered, like an onion. In the final few seconds, as the core continues to collapse, all the atoms get squeezed against each other, which creates a colossal amount of energy and pressure. This sends a shockwave of energy across the different shells.
At this point, the star goes supernovae, spraying every element it created into endless space!
Formation Of Elements Heavier Than Iron
Remember the last few seconds and the shockwave we just mentioned? As the star is dying and exploding into a supernova, it releases a huge amount of energy (the temperature rises to billions of Kelvins) and a very dense cloud of neutrons.
R-process
These neutrons interact with the atoms of the already formed elements. They undergo a series of fusion and fission to form elements up to Uranium, as well as a few transuranic elements like Curium, Californium, and Fermium. This entire process of rapid neutron capture or r-process takes place in less than a second. Elements like gold, platinum, and silver are so rare and expensive because it takes a dying star to create them!
S-process
Another common route is through a much slower process of neutron capture, also known as the s-process. This could take place in different fusion layers of a star or within a neutron star that has ample neutrons and the right conditions for the capture. The mechanism for the s and r-processes is the same.
P-process
The nucleus of an element captures the neutrons and changes into its isotope. If the isotope formed isn’t stable the nucleus undergoes beta decay to form the next stable element. Thus, all the elements that we know of above iron and up to uranium were produced by this continuous process. Another form of nucleus growth is via proton capture or p-process. To read more click here.
This is true for all elements, except for Technitium and Promethium, which don’t have stable isotopes that could last long enough for us to find here. All the elements after uranium are man-made and radioactive with short half-lives.
This leads to another question… How did the elements created by an exploding star end up here on Earth?
Delivery To Earth
The universe is a gargantuan recycling bin; it recycles and reuses every piece of matter that was once created by a process called chemical enrichment. Millions of galaxies, stars, and planets were formed and will continue to form using the same primordial matter left over after the Big Bang.
The young universe was ¾ Hydrogen and ¼ Helium, while the rest of the matter was negligible. However, after billions of years of burning and exploding, the universe now has a (drumroll please) 2% composition of other elements! This might seem unimpressive, but on a cosmic scale, it’s plenty!
The elements expelled into space after the death of a star eventually find their way to new star-forming regions, where young stars are beginning their journey. Due to gravity, some of the dead star stuff becomes a part of the next generation of stars.
After those stars die, the matter is again returned to the cosmos. This cycle continues over and over for eons and millennia. A similar thing happened when our own solar system was forming. A large proportion of it ended up creating our beloved big ball of fire, the sun. The remaining stardust orbiting the sun, however, eventually clumped up to form asteroids and planets, including our home—Earth.
Conclusion
Believe it or not, but all the atoms in our bodies are older than the solar system itself! They were created in a series of events following the one event that started it all 13.8 billion years ago. The gold in our jewelry and the zinc in our batteries were created in the final moments of a star’s life. The oxygen and carbon in our soda, the iron in our blood, and the calcium in our teeth were forged in the smoldering heart of a star. The cosmos is truly within all of us.
References (click to expand)
- The early universe - CERN. The European Organization for Nuclear Research
- Lecture 44: The First Three Minutes. The Ohio State University
- Brief History of the Universe. The University of California, Los Angeles
- Where do atoms come from? - UCSB Science Line. The University of California, Santa Barbara
- Jefferson Lab (2012). The Origin of the Elements. Youtube
- Lecture 44: The First Three Minutes. The Ohio State University
- The Life Cycles of Stars - Imagine the Universe! - NASA. The National Aeronautics and Space Administration
- Zaikowski, L.,& Friedrich, J. (2008). Chemical Evolution across Space and Time: From the Big Bang to Prebiotic Chemistry (ACS Symposium Series, 981). American Chemical Society
- Caltech Astro (2020). The Explosive Origin of the Elements - Andrew Emerick - 08/07/2020. Youtube
- The Cosmic Origin of the Elements - Imagine the Universe!. The National Aeronautics and Space Administration