Ionizing radiation, unlike non-ionizing radiation, can cause chemical bonds to break by removing electrons from atoms or molecules.
Do you ever find yourself wondering how an X-ray machine gives us the images of our bones and soft tissues? Or how a microwave oven warms up frozen food in mere minutes?
This “invisible light” that seemed to pass through screens baffled scientists for years, until several hypotheses and experiments later, when the term “radioactivity” was coined.
Recommended Video for you:
What Is Radiation?
Radiation is simply energy transmitted in the form of electromagnetic waves, particles through a medium, or even through a vacuum. However, this energy emitted from a source can be observed in two different forms–ionizing and non-ionizing radiation.
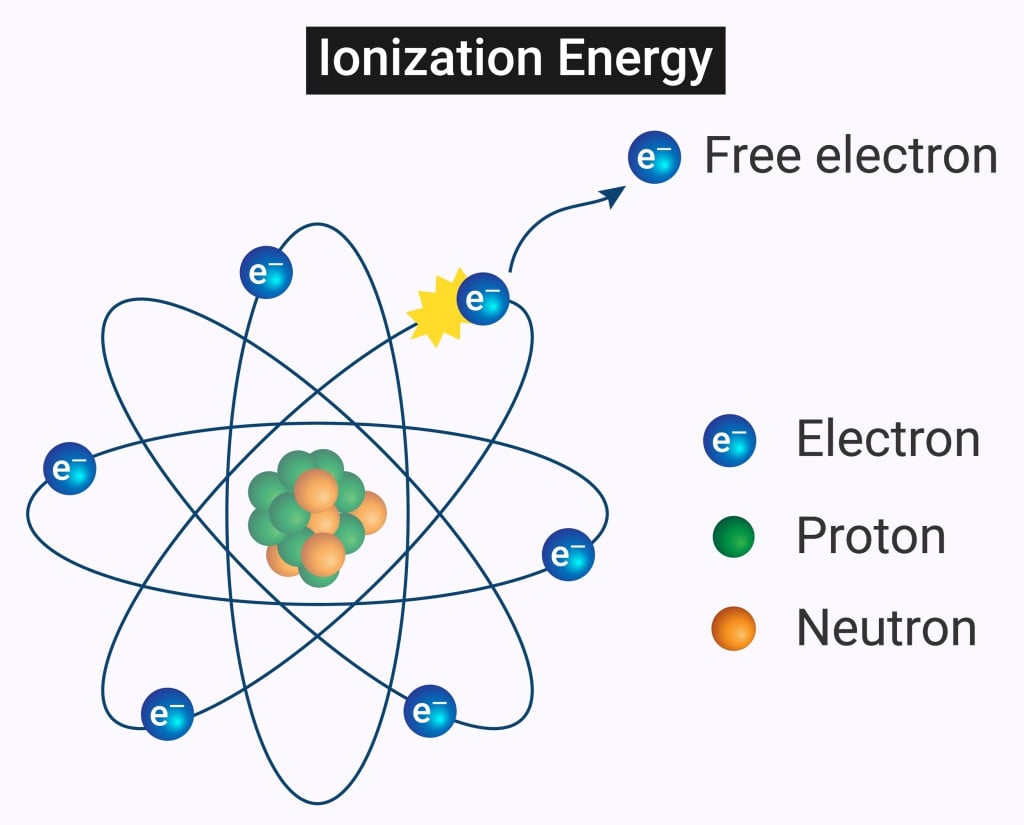
Ionizing radiation is more energetic than non-ionizing radiation. Unlike non-ionizing radiation, ionizing radiation can cause chemical bonds to break by removing electrons from atoms or molecules. This causes the atoms or molecules to become ionized.
The Discovery Of Radiation
During the process of researching ionizing radiation in 1895, Wilhelm Rontgen conducted an experiment in his lab where he was examining cathode rays. He wanted to test whether these rays could pass through objects or not, as he had observed some light coming from a nearby screen placed in the experimental setup.
While performing several experiments, he also created an X-ray machine that showed the skeletal structure of his wife’s hand. This “invisible light” that was somehow able to pass through thick bones and layers of muscles to produce images of internal bones baffled him, so he coined the term “X-rays” to designate it as something new or unknown.
How Was The Term “Radioactivity” Coined?
In 1896, Henri Becquerel realized that uranium salts generally emit this invisible light. He hypothesized that it could be due to exposure to the sun, but through further experimentation, he accepted that it was the molecule itself giving off this light. His PhD student, Marie Curie, named this phenomenon Radioactivity. She later discovered radioactive elements – thorium, polonium and radium—and went on to win two Nobel prizes for her revolutionary work in this field.
She even played a crucial role in World War I by creating X-ray machines that could locate bullets inside soldiers’ bodies.
What Are Isotopes And Radioisotopes?
Isotopes are variations of an element that have slightly different masses. One can imagine them as twins, triplets, quadruplets, etc., so while they share a similar identity, their age, weight and other characteristics might differ from each other.
Isotopes of an element have the same number of protons in their nuclei, but the number of neutrons differ for every version (isotope) of that element.
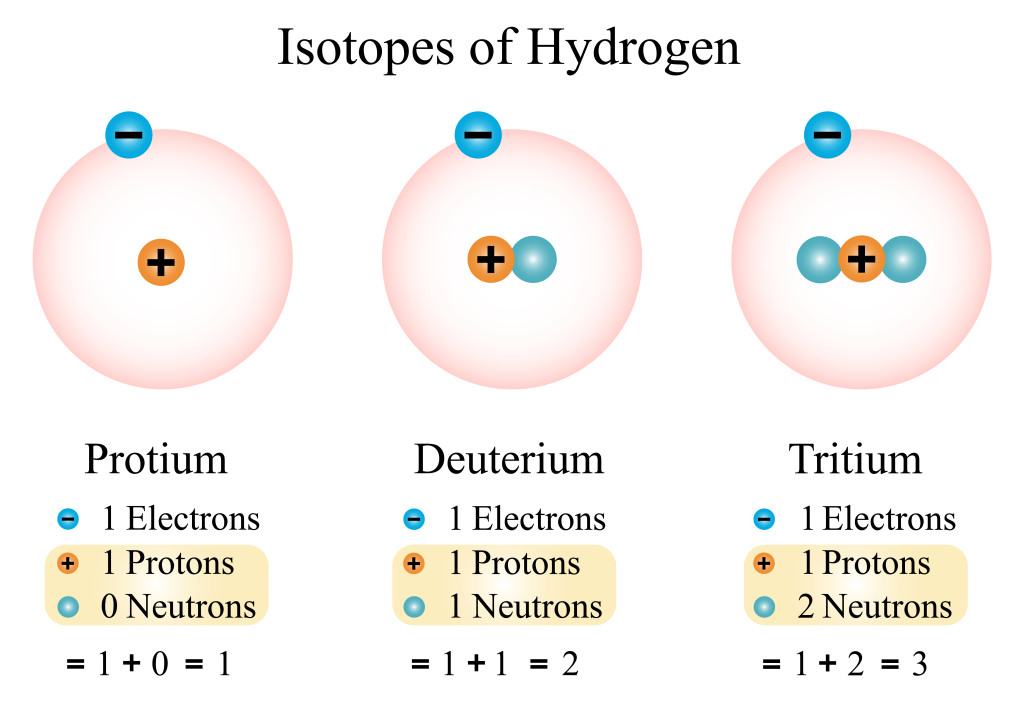
In this image above, we see the isotopes of hydrogen, where although they have the same atomic number (1), their atomic mass number differs (1, 2, 3). Every isotope has unique properties and characteristics that enable a wide range of uses in scientific and industrial fields.
Stable And Unstable Isotopes
There also exist stable and unstable isotopes. The nuclei of a stable isotope remains intact for a long period of time. This is due to a balance in the number of protons and neutrons, making their energies stable. The isotope of carbon – 12C and nitrogen – 14N are some examples of stable isotopes commonly found in nature. Stable isotopes are predominantly used in isotope analysis, research, and medicine.
Unstable isotopes, on the other hand, are also called radioactive isotopes or radioisotopes. These isotopes have an unstable nuclei that undergoes spontaneous decay, while emitting radiation in the process. They show an imbalance in the number of protons and neutrons, so they are energetically unstable. The radiation emitted by unstable isotopes is quite harmful if a person is exposed to it for prolonged periods. These isotopes also have substantial importance in nuclear power generation.
What Does Radioactivity Mean?
When an unstable nuclei decays, it releases a certain amount of energy by emitting particles, including electrons, protons, and neutrons. This process of releasing energy is called radioactivity.
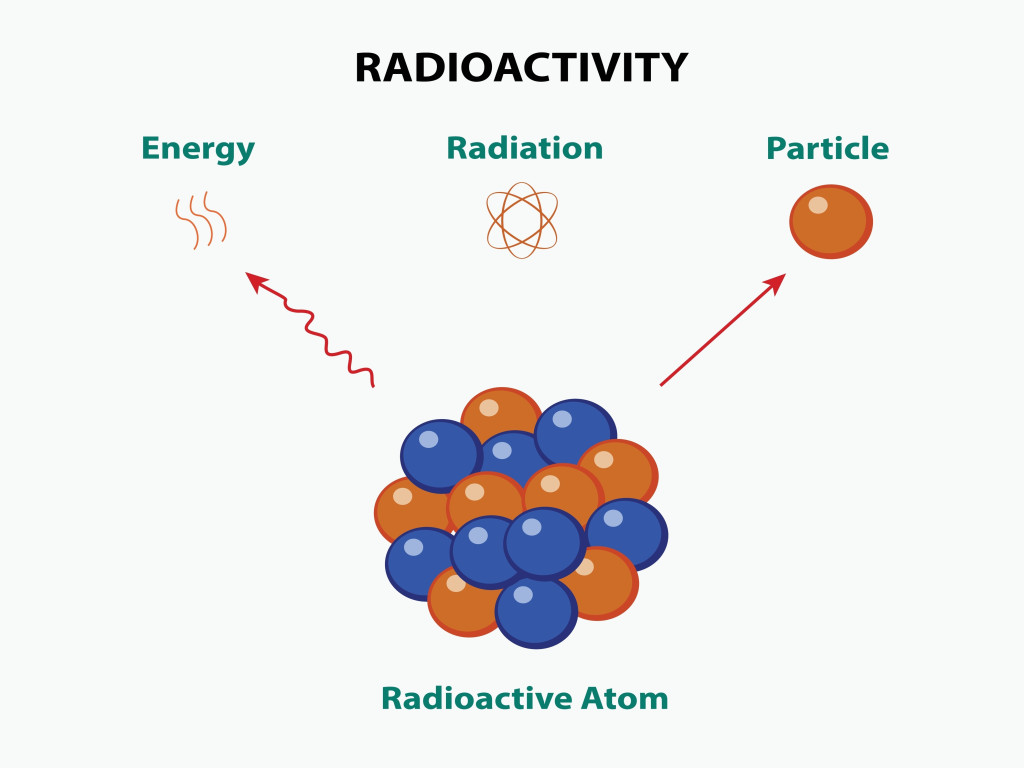
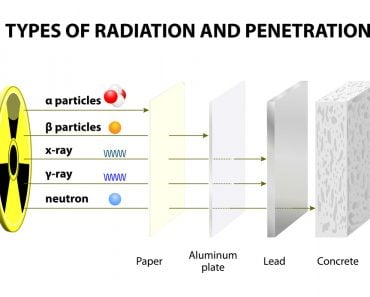
The particles emitted are highly energetic and can remove electrons from other atoms and molecules. The highly energetic and charged particles removed from the atoms are known as ions. Depending on the number of particles released, radiation can be classified into three types: alpha, beta and gamma decay.
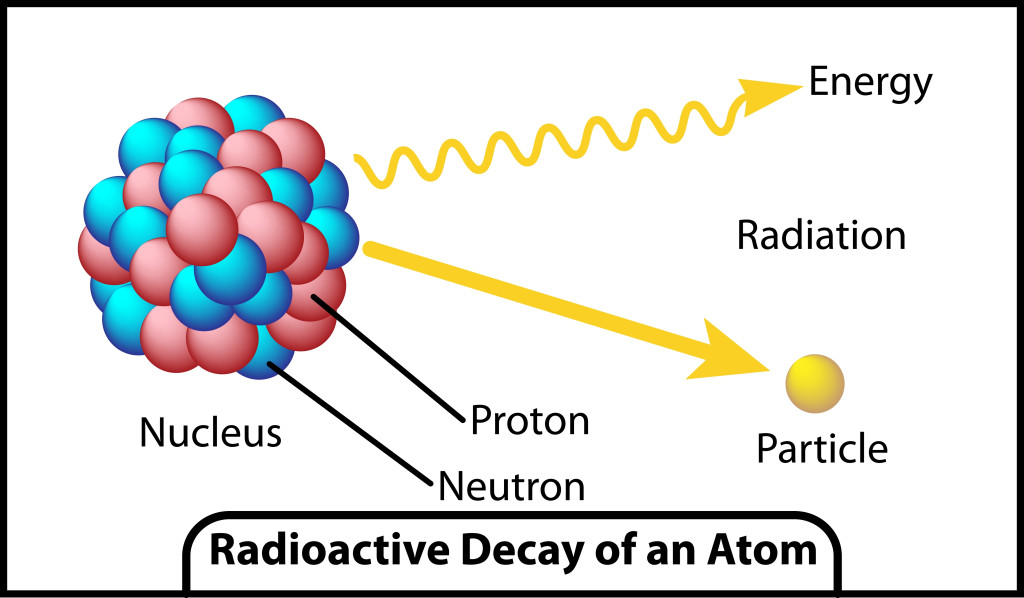
The properties of the alpha, beta and gamma particles being released can be understood from the image above. One should note that radiation caused by all three types of particles have enough energy to cause the ionization of atoms/molecules.
Why Do Unstable Isotopes Emit Only Ionizing Radiation And Not “Regular” Radiation?
Since radioactive decay allows for the release of only high-energy particles, unstable isotopes emit ionizing radiation, rather than “regular” radiation.
However, it is necessary to note that some unstable isotopes, like Carbon-14 and Phosphorus-32, being radioactive, emit non-ionizing radiation. These isotopes are extensively used in medicine.
The ionizing radiation, while interacting with matter, can transfer energy to atoms or molecules, thereby causing the emission of electrons from their respective orbits. This leads to the initiation of reactions that could possibly harm the cells of a human body, ultimately building into cancer cells and tumors.
It is extremely essential to remember that although light is also a form of radiation – non-ionizing radiation. Other examples include radio waves, microwaves and visible light of course! These types of radiation are not harmful and show negligible biological effects on a living thing. Medical diagnostic techniques use small amounts of “unstable” isotopes to accurately detect cancer cells in a body before the tumor can become malignant. So, while radiation can be an intimidating word, and a powerful form of energy, it can also be harnessed to do incredible things.
References (click to expand)
- DOE Explains...Radioactivity.
- What are Radioisotopes? | ANSTO.
- Mousseau, T. A., & Møller, A. P. (2020, May 8). Plants in the Light of Ionizing Radiation: What Have We Learned From Chernobyl, Fukushima, and Other “Hot” Places?. Frontiers in Plant Science. Frontiers Media SA.
- Morgan, W. F., & Sowa, M. B. (2007, March). Non-targeted bystander effects induced by ionizing radiation. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. Elsevier BV.