Table of Contents (click to expand)
Aging is the progressive loss of the body’s ability to maintain itself. Recent studies have found that some synthetic drugs have chemical compounds that can delay the aging process. Such medicines activate enzymes that provide more energy to our cells or stimulate our body to repair itself better and fight off free radicals.
When I was a kid, I couldn’t wait to grow older and live without rules and homework. My mom always told me that I’d wish I were younger once I became old… and I’m starting to understand why she said that. Growing old comes with many issues.
Random back pain, lower energy levels, body stiffness, and a decline in brain cognition are all symptoms of growing old. Aging is a natural phenomenon and it’s a part of life. However, what if there was a way to slow it down so we could cling to our fruitful youth just a little longer?
Well, before we try to slow aging down, let’s try to understand what it is.
Recommended Video for you:
What Is Aging?
Aging is the progressive loss of the body’s ability to maintain itself. This leads to a functional decline in our cells and tissues. The hallmarks of aging are genomic instability, loss of protein homeostasis, mitochondrial dysfunction, poor cell-cell communication, and stem cell exhaustion.
But what causes this? There are a few factors to consider. For starters, all cells divide. That’s how we grow. Each time the cell divides, its DNA is replicated too. DNA replication is a complex procedure that can be error-prone, at times. Errors in DNA replication cause mutations that change DNA, which subsequently affect cellular functioning. DNA mutations also occur naturally because we’re constantly exposed to UV radiation from the sun and other environmental radiation sources.
Furthermore, each time DNA is replicated, a small portion is lost. However, our cells are equipped to take on this loss with the help of telomeres. These are repetitive DNA sequences located at the end of the chromosomes. Their role is to provide the excess DNA with a buffer that our cells can afford to lose. However, once they run out…. they’re gone!
Controlling DNA replication is not something in our hands… yet. However, we can control aging’s most progressive factor – free radicals. Free radicals are highly reactive oxygen molecules made by our bodies during normal metabolic processes. The human body needs oxygen to breathe and carry out numerous biochemical processes. In doing so, oxygen is broken chemically to release energy and we are left with oxygen molecules that are missing electrons.
Examples of such free radicals include hydroxyl (OH), superoxide (O2), nitric oxide (NO), nitrogen dioxide (NO2), peroxyl (ROO) and lipid peroxyl (LOO). These free radicals are either reactive oxygen species (ROS) or reactive nitrogen species (RNS) and they are commonly made in the mitochondria.
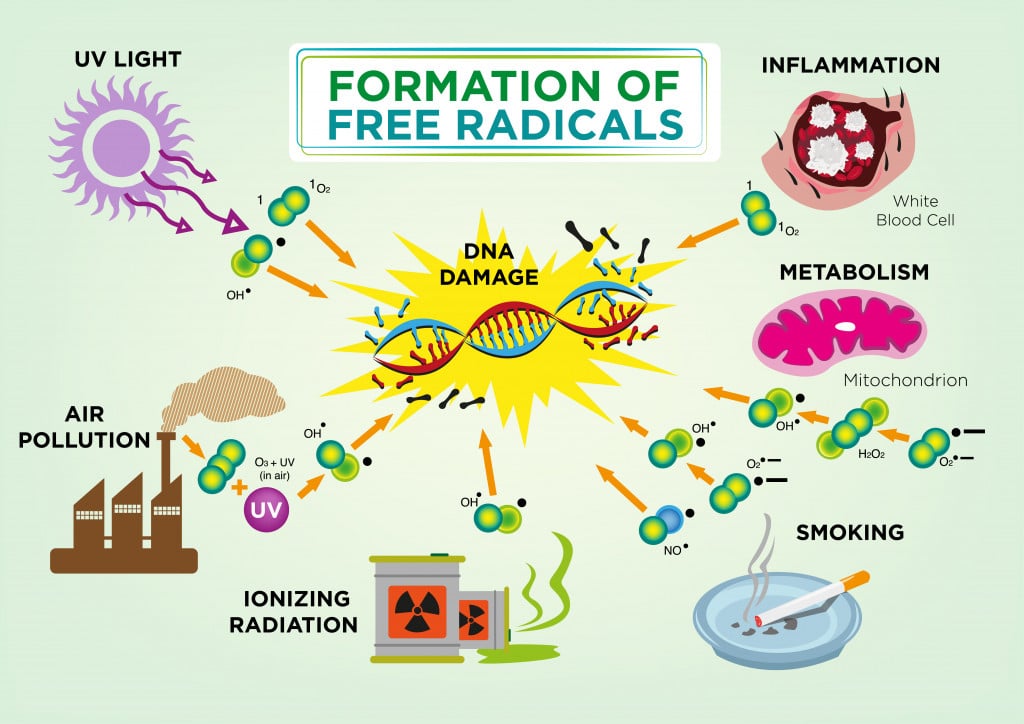
As you might remember from high school chemistry, atoms missing electrons will look for a partner to share or take an electron from. That makes them reactive, as they are looking to mingle with other fellow elements. These free radicals react with our DNA, lipids and proteins, and damage them, causing wear and tear to our cells.
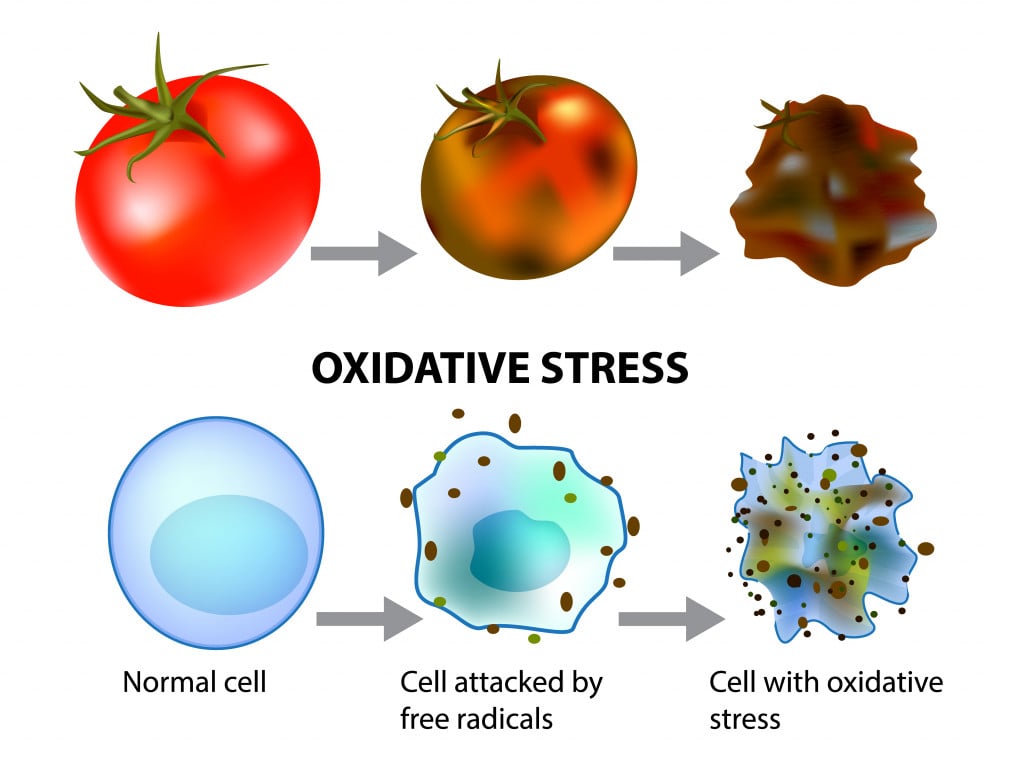
Free radical generation is a normal phenomenon, but unhealthy lifestyle habits like smoking and stress can increase their generation rate. The human body has ways to repair itself, although, with time, its self-repair efficiency lessens. This, coupled with increased free radical generation, physically wears down the body until, voila! – you find yourself growing old.
Once we knew the two main causes aging, we tried to slow it down by targeting them. There are a few ways to do that.
Telomere Maintenance
After every DNA replication cycle, the telomeres shorten. Once the telomere almost reaches the end, the cell’s replication cycle stops and we get replicative senescence. That’s the term used to describe the stage where cells stop trying to grow themselves. If we found a way to make the telomeres longer, we could find a way to make cells live longer.
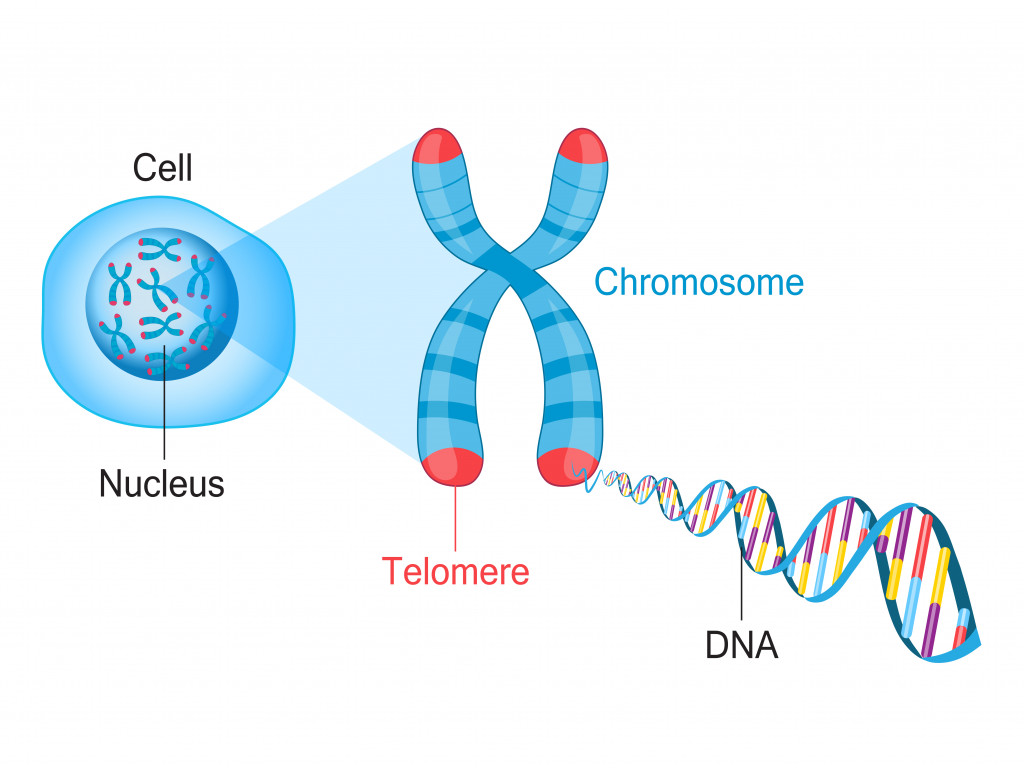
That’s when the enzyme telomerase comes into play. This is an enzyme some stem cells have that can continue adding repetitive DNA sequences to telomeres and delay senescence. Stem cells have the special ability to make telomerase because they preserve telomeres for future generations. However, the activity of telomerase is strictly controlled. Improper telomerase activity may cause genetic instability and even disease, as uncontrolled DNA changes can affect the body in drastic ways.
By using stem cell therapies and genetic modification, scientists are trying to manipulate telomerase so it dances to their tune. By controlling how and when telomeres are lengthened, it will be possible to delay cell death and push cells to keep multiplying for longer.
That being said, not everyone is comfortable modifying their DNA. Plus, it will still take a while to get there with this technological theory. Isn’t there something easier like an anti-aging pill?
Anti-aging Drugs
Recent studies have found that some synthetic drugs contain chemical compounds that can delay the aging process. Such medicines activate enzymes that provide more energy to our cells or stimulate our body to repair itself better and fight off free radicals.
Take the famous drug, aspirin, which is used to relieve pain during headaches and prevent blood clots. Later studies found that it also prevents nitric oxide formation, the cell-damaging free radical, and increases telomerase activity.
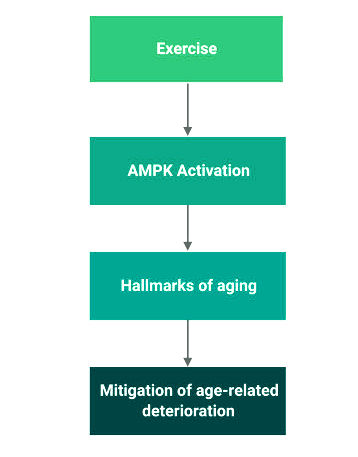
Metformin, a drug familiar to diabetics, does more than help cells become more sensitive to insulin. It activates adenosine monophosphate-activated protein kinase (AMPK), an enzyme that plays an essential role in many cellular pathways, so that it can control a body’s metabolism.
Humans use Adenosine Triphosphate (ATP) as energetic currency in their cells. When a cell runs low on ATP, AMPK is activated. This enzyme switches on pathways that give cells an energy boost as it increases glucose uptake and fatty acid breakdown. The energy surplus is directed towards cell repair and survival processes. AMPK activation also suppresses other energy-demanding processes that aren’t necessary at the moment, like lipid synthesis.
Check out this video from WIRED UK for more info about Metformin as an anti-aging drug.
However, just as some people aren’t comfortable with DNA modifications, others don’t want to take medicine for the rest of their life. With that in mind, let’s move on to some other ways that aging can be delayed.
Caloric Restriction
This method isn’t for the mentally weak or hungry food lovers. Caloric restriction deprives your body of nutrients, but not to the point where you become malnourished. This intervention slows age-related changes and delays age-related disorders. Its mechanism is similar to Metformin, where the body is pushed into survival mode. The key regulator in this approach is also AMPK.
A low-calorie diet, a.k.a. a glucose-starved body, activates AMPK. This nutritional stress stimulates AMPK to activate cell survival pathways, while halting the non-essential ones.

Low-calorie diets = less food consumed = less metabolic processes required to break down that food. If a person’s metabolism is lowered, then so is oxygen breakdown during biochemical processes. That’s how caloric restriction also lessens free radical generation, which reduces oxidative stress on the cells. However, not everyone is a fan of self-starvation, particularly as a long-term lifestyle choice.
Exercise
Exercise is something well known to have anti-aging effects, while also improving health. It fights several aging hallmarks.
Exercise strengthens muscles and improves endurance, which slows down the chance of developing age-related disorders like diabetes and osteoporosis.
Exercising requires cells to consume more energy, so you can probably guess what’s activated to provide that — AMPK! It is activated in the skeletal muscle cells and liver cells to increase glucose uptake. On top of that, AMPK also activates defensive antioxidant proteins, such as nuclear factor erythroid 2-related factor 2 (Nrf2). This antioxidant protein activates antioxidant genes that code for antioxidant enzymes like catalase and superoxide dismutase, which detoxify and remove free radicals.
Additionally, long-term exercise affects telomerase activity, so it’s able to increase telomere length. It also activates DNA repair proteins, essentially reducing mutations and DNA damage.
Exercise is a very effective way to slow aging and increase one’s life span, as it actively counters all the hallmarks of aging.
Reducing Stress
Stress is a nearly unavoidable part of our lives. Getting older is stressful in itself. We lose cognitive and functional abilities, cope with loss, money problems and health issues. Ironically, it becomes a loop. We become stressed with age, and the more the stress we take on, the quicker we age.
Chronically stressed people age rapidly. Stressed people tend to have hyperimmune responses, where the immune cells release pro-inflammatory cytokines. These molecules increase oxidative stress in the mitochondria of other cells in the body. Free radical generation increases, which causes cell damage, and people suffering from long-term stress also have faster rates of telomere shortening. In fact, one study found that people suffering from mood disorders had shorter telomere lengths. Stress, therefore, makes cells reach senescence quicker. Too much stress even makes people prone to age-related diseases.
By removing or avoiding stress, we reduce its harmful aging-associated effects and improve our lifespan. Studies show that meditation can help reduce stress levels and slow cellular aging. Exercise is another excellent way to cope with stress-related cellular aging.

Getting regular sleep also helps in coping with stress and it allows the body time to rest and repair. Sleep-deprived people suffer stress-related disorders more than their sleeping counterparts. One study found that sleep-deprived people have higher ROS levels in their cornea, contributing to quicker eye aging. In other words, it’s time to put the phone away at night.
Conclusion
It’s no wonder that experts worldwide focus so heavily on living a healthy, balanced lifestyle with a good diet and exercise. Many cellular changes happen as we grow older, but there are ways to combat them.
Our genes are not something we can control yet, but we can do our best to maintain our oxidative stress levels and reduce free radical production. Eating fruits and vegetables rich in antioxidants like Vitamins E, A & C and plant polyphenols like quercetin help to destroy free radicals. An antioxidant-rich diet is a terrific solution to oxidative stress.
We can’t permanently stop aging, but it’s clearly possible to slow it down. The goal of anti-aging is to improve our health and longevity. Anti-aging doesn’t turn a 50-year-old into a 15-year-old; instead, it helps make the 50-year-old feel they’re 15 again!
https://biosignaling.biomedcentral.com/articles/10.1186/s12964-021-00706-1
References (click to expand)
- Carapeto, P. V., & Aguayo-Mazzucato, C. (2021, May 13). Effects of exercise on cellular and tissue aging. Aging. Impact Journals, LLC.
- Richter, E. A., & Ruderman, N. B. (2009, February 11). AMPK and the biochemistry of exercise: implications for human health and disease. Biochemical Journal. Portland Press Ltd.
- Herzig, S., & Shaw, R. J. (2017, October 4). AMPK: guardian of metabolism and mitochondrial homeostasis. Nature Reviews Molecular Cell Biology. Springer Science and Business Media LLC.
- Yegorov, Y. E., Poznyak, A. V., Nikiforov, N. G., Sobenin, I. A., & Orekhov, A. N. (2020, July 7). The Link between Chronic Stress and Accelerated Aging. Biomedicines. MDPI AG.
- Lavretsky, H., & Newhouse, P. A. (2012, September). Stress, Inflammation, and Aging. The American Journal of Geriatric Psychiatry. Elsevier BV.
- Nimse, S. B., & Pal, D. (2015). Free radicals, natural antioxidants, and their reaction mechanisms. RSC Advances. Royal Society of Chemistry (RSC).
- Hadley, E. C., Lakatta, E. G., Morrison-Bogorad, M., Warner, H. R., & Hodes, R. J. (2005, February). The Future of Aging Therapies. Cell. Elsevier BV.
- Kapoor, V. K., Dureja, J., & Chadha, R. (2009, September). Synthetic drugs with anti-ageing effects. Drug Discovery Today. Elsevier BV.