The ice, upon coming in contact with boiling oil, quickly melts and changes to steam. The large volume of steam that is formed will then rise, carrying some of the oil along with it as smaller droplets, which burst in the air… and voila! Armageddon.
It’s hard to deny that deep-fried anything tastes amazing! Fried food is so popular that it has seemingly seeped into every meal of the day. For breakfast, you have hash browns, fried chicken for lunch, and French fries for a snack. And for dinner… how about some deep-fried ice?
The answer is and always should be a definite NO.
Here’s why.
Recommended Video for you:
What Happens If You Deep-fry Ice?
Deep-frying anything involves heating oil to high temperatures and then tossing in your item of choice. However, if you choose to throw ice into the frier, you would get an explosion.
First off, PLEASE DO NOT TRY THIS AT HOME. You can do a quick online search and find tons of videos of people throwing ice into pots of boiling oil. What follows is a huge explosion, with oil and steam bubbling and spewing everywhere. All of this happens in a matter of seconds, so I cannot stress how important it is that you satisfy yourself with video evidence, rather than setting your home and yourself on fire.

So, what causes this explosion? Well, when the ice comes in contact with boiling oil, it quickly melts and changes to steam. The steam rises and makes a run for it, dragging some of that hot oil along with it.
Now, let’s get down to the science of this phenomenon.
The Chemistry Of Combustion
We know that boiling oil is dangerous because it’s hot, but ice isn’t an explosive, so what makes this combination so deadly? To understand this, let’s state some facts about the two substances.
Ice has a temperature of 0°C (32°F). Oil for deep frying sits at temperatures between 150°C to 200°C, depending on the type of oil. You can already see the huge 150°C temperature difference between ice and oil, but why is that important?
Matter generally exists in three states: solid, liquid and gas. When any substance changes from one state to another, the particles inside vibrate. This vibration can either increase or decrease, depending on which state the material in question is changing to. Higher temperatures will cause the particles to vibrate faster. You can imagine that boiling oil will have particles vibrating at a chaotically rapid pace.
And then you add another element to this chaos—ice. Upon hitting the oil, that large 150°C temperature difference is quickly transferred to the ice, which melts and becomes water.
We know that water and ice don’t mix. As the denser of the two, water sinks to the bottom, but in this case, the temperature is still so high that the water quickly turns to steam. As you know, when any substance becomes a gas, its volume increases.
Now, let’s cumulate these points and paint a picture.
- Ice melts to water.
- Water sinks to the bottom of the oil.
- As ice turns to steam, it expands and escapes.
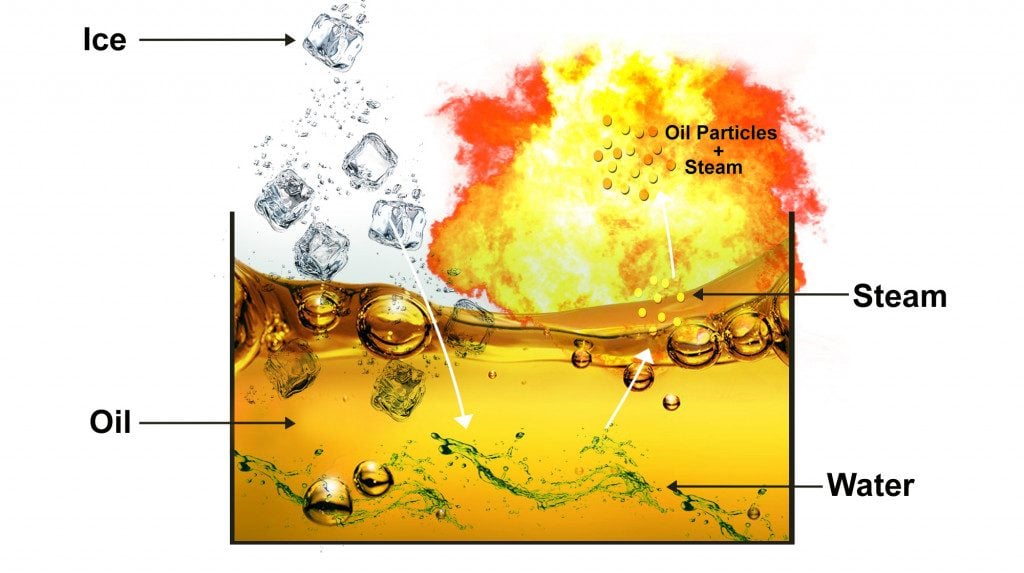
The large volume of steam now rises, carrying some of the oil along with it as smaller droplets, and bursts into the air. The remaining oil bubbles and spills over. Worse yet, some of this particulate matter of steam and oil will catch fire in the air, leading to complete chaos in the kitchen!
How Does It All Catch Fire?
You may be wondering what causes the fire in this situation. We’ve all left oil in the pan a few seconds too long and the most we’ve probably seen is some smoke, so where does the fire come from?
Different oils have different temperatures they need to reach before they can catch fire. Click here to find out more about these boundaries. Essentially, when any oil reaches its flash point, the vapors it exudes are flammable.
When the oil and vapors rise out because of the steam from below, the vapor particles in the air can catch fire, creating an explosion. Usually, the temperatures needed to reach this are quite high, so a fire breaking out after chucking an ice cube in a frier is rare. The temperatures required to keep such a fire going, the fire point, is even higher, which is why after the explosion, the fire dies down on its own.
In the case of ice and hot oil, the amount of ice that falls in matters. One or two cubes of ice will cause some bubbling, and maybe some spilling and splatter, but not an explosion. However, if you’ve seen videos online, you know that with more ice, the experiment turns into a recipe for disaster.
Why Doesn’t Ice Have Any Chill?
Naturally, one wonders why throwing a large amount of ice into something doesn’t cool the thing down. Obviously if we’re talking about throwing a large bag of ice into a tiny beaker of boiling oil, the reaction would be severely anti-climatic.
But without that large volume of ice, the much larger difference in temperature between the two materials crushes any cooling effect that the ice brings. Ironically, ice is just not cold enough to battle boiling oil. Now, what about dry ice, a much colder cousin… would that do the trick?
What Happens When You Deep-fry Dry Ice?
Dry ice is the solid form of carbon dioxide, boasting a temperature of -78°C (-109°F), making it much colder than ice. Logically, you could imagine one of two things to occur when you deep-fry it.
- Since the temperature difference here is even larger, about 400°F, the heat energy transferred from dry ice to the oil is even larger. This results in an even bigger explosion, as compared to ice.
- Since the dry-ice temperature is much lower, it can bring down the temperature of the oil, thus preventing an explosion.
Surprisingly, neither of these two are correct! Deep-frying dry ice causes a milder reaction, wherein the oil bubbles and spatters a bit, but nothing like the case with ice. This is because dry ice does not have an intermediate liquid form. It quickly changes to gas upon touching hot oil and then immediately escapes.
Therefore, it does not carry any of the oil from below while it bursts out; it just escapes off the top. While this reaction also occurs quite rapidly and causes some bubbling, no explosion occurs.
Where Did Deep-fried Ice Cream Come From?
Intuitively, deep-fried ice cream seems impossible after reading this article. However, remember that the tastiest part of deep-fried ice cream is the coating around it. This coating can be composed of cornflakes, dough or batter.

This coating is not just for the taste. It’s the coating that gets deep-fried here, not the ice cream. The center of ice cream does not come in contact with the hot oil, and remains somewhat cold.
So Ice And Hot Oil Are Mortal Enemies?
To be on the safe side, yes, these two substances should be considered enemies, but ice and oil at regular temperatures can be friends too! If you’ve seen the viral video of people using ice to remove excess oil from dishes, you will see why!

This occurs because the oil and fat in these dishes quickly solidify at the low temperature of ice, allowing us to remove them easily.
The bottom line is to not try this at home, ever. That said, if it were to accidentally happen, the best thing would be to stay as far away as possible and call for help. Even without causing a fire, the oil splatters can cause some serious burns!
References (click to expand)
- Liu, J., & Chow, W. (2017, August). Fire hazards of introducing water and ice into hot oil in open kitchen. Journal of Fire Sciences. SAGE Publications.
- The Chemistry of Combustion. Florida State University
- Siu, S. K., Phillips, C. R., & Chen, E. C. (1977, January 1). The Continuous Spilling of Hot Oil on Ice. Journal of Canadian Petroleum Technology. Society of Petroleum Engineers (SPE).