Table of Contents (click to expand)
The hydrogen bonding between sugar and water molecules make sugar solutions ‘sticky’. The extensive H-bonding increases the cohesion and adhesion of the solution, which, in turn, results in its stickiness.
Sugar syrup, maple syrup, honey, cotton candies… all these sweet delicacies have two things in common: one, they are all products of sugar being dissolved in water, and two, they are all STICKY!
Sugar by itself is just a sweet crystal, and water isn’t sticky either, so why do water and sugar, when combined, produce a sticky gooey mess?
In order to find how these seemingly mundane substances transform completely when mixed together, we have to dive deep into their molecular structure.

Recommended Video for you:
A Closer Look At Sugar And Water
The Structure Of Sugar
‘Sugar’ is an umbrella term used to describe a lot of different carbohydrates, but for now, let’s use the term to refer to our very own ‘table sugar’, aka ‘sucrose’.
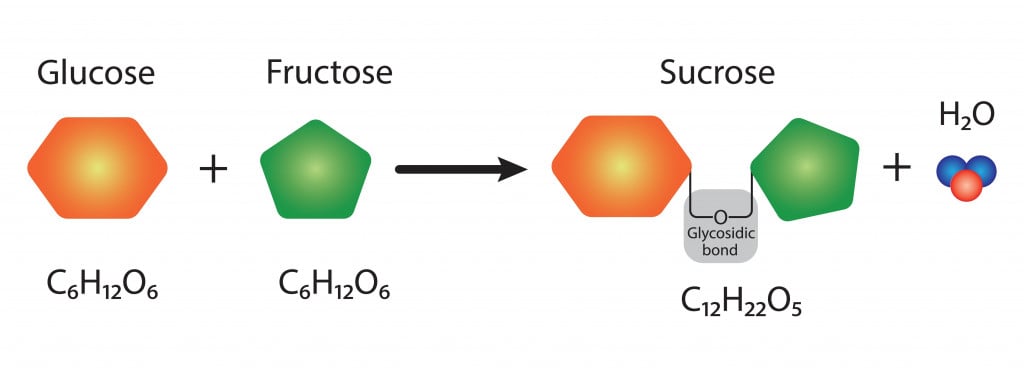
Sucrose belongs to a class of molecules called carbohydrates, since it is made of carbon, hydrogen, and oxygen atoms. It consists of 12 carbon, 22 hydrogen, and 11 oxygen atoms, hence the chemical formula C12H22O11.
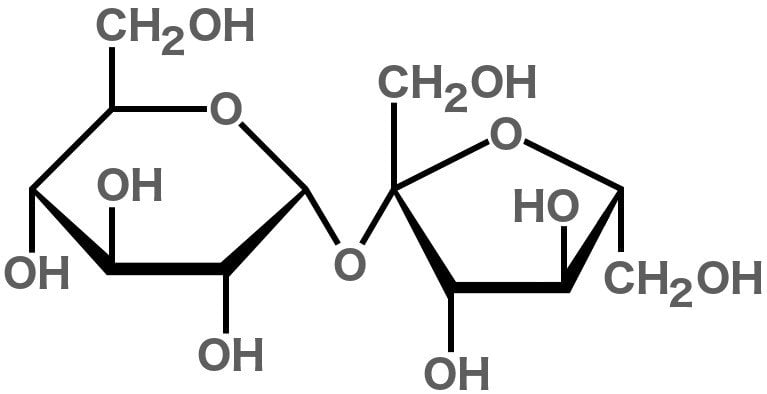
Sucrose is considered a ‘disaccharide’ because it is formed by joining two monosaccharides (simple sugars)—glucose and fructose.
The Water Molecule
Water (H2O) is a molecule with which we are all familiar. It consists of two hydrogen atoms covalently bonded to an oxygen atom. Even though water looks like a simple molecule, its physical and chemical properties are extremely complex.
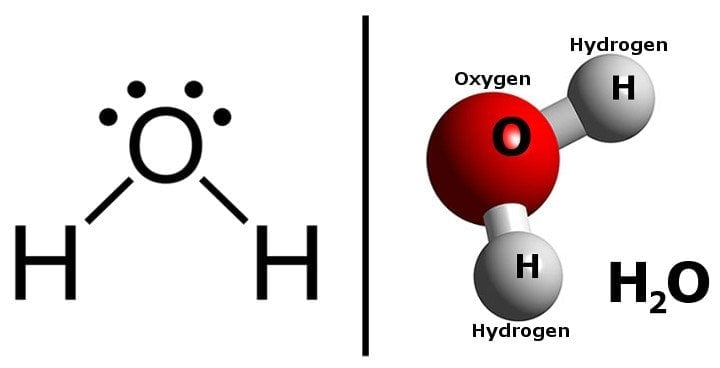
Comparing the two structures, we can see that water and sugar have something in common; both have O-H bonds and both of the molecules are formed by covalent bonding.
These are the main factors resulting in the stickiness of sugar solutions. The covalent O-H bonds participate in something called ‘Hydrogen Bonding’, which provides sugar with all the amazing properties we witness and benefit from.
Covalent Vs Ionic Molecules
Every atom’s ultimate aim is to attain stability, which is obtained by having a completely filled valence shell. To achieve this electron configuration, atoms take different approaches;
- Ionic Bonding: This bond is formed by the transfer of electrons between atoms. It’s like giving your extra pencil to a friend who doesn’t have one. Some atoms donate their extra electrons to other atoms, who accept these to attain stability, thus forming an ionic molecule, e.g., salt;

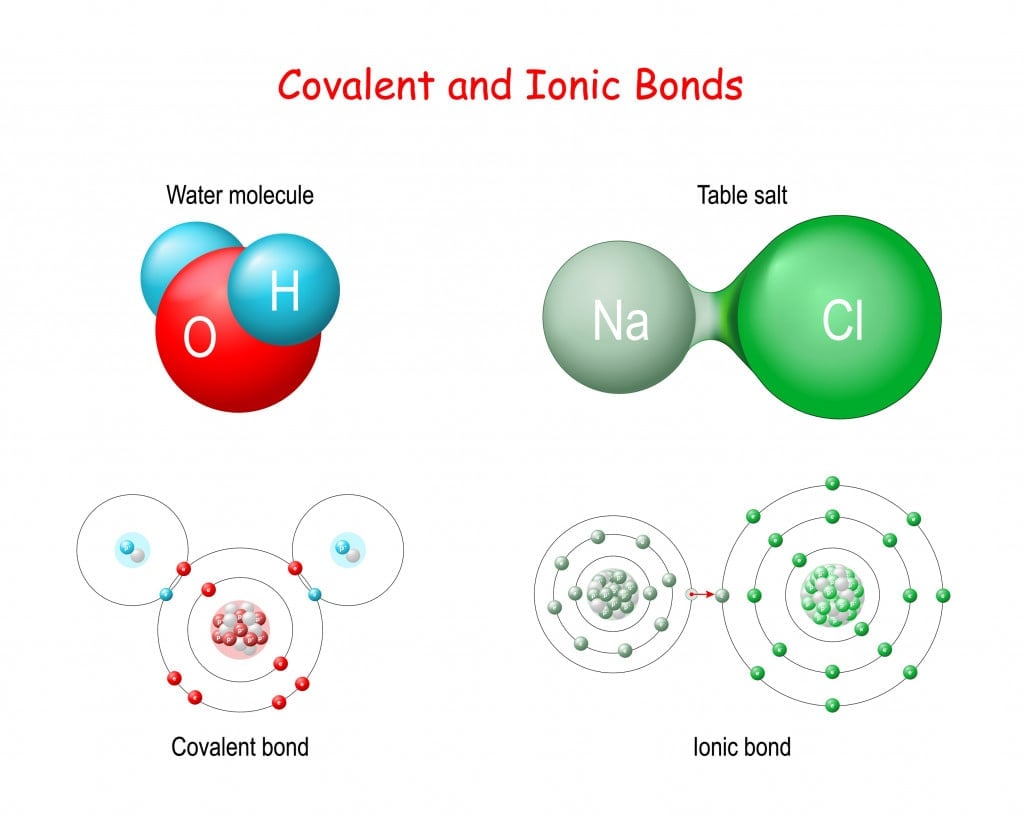
- Covalent Bonding: This bond is formed by the sharing of electrons between atoms. In this case, two bonding atoms share a pair of electrons, and this results in the formation of a covalent molecule, e.g., sugar and water.

Ionic and covalent molecules behave differently in water:
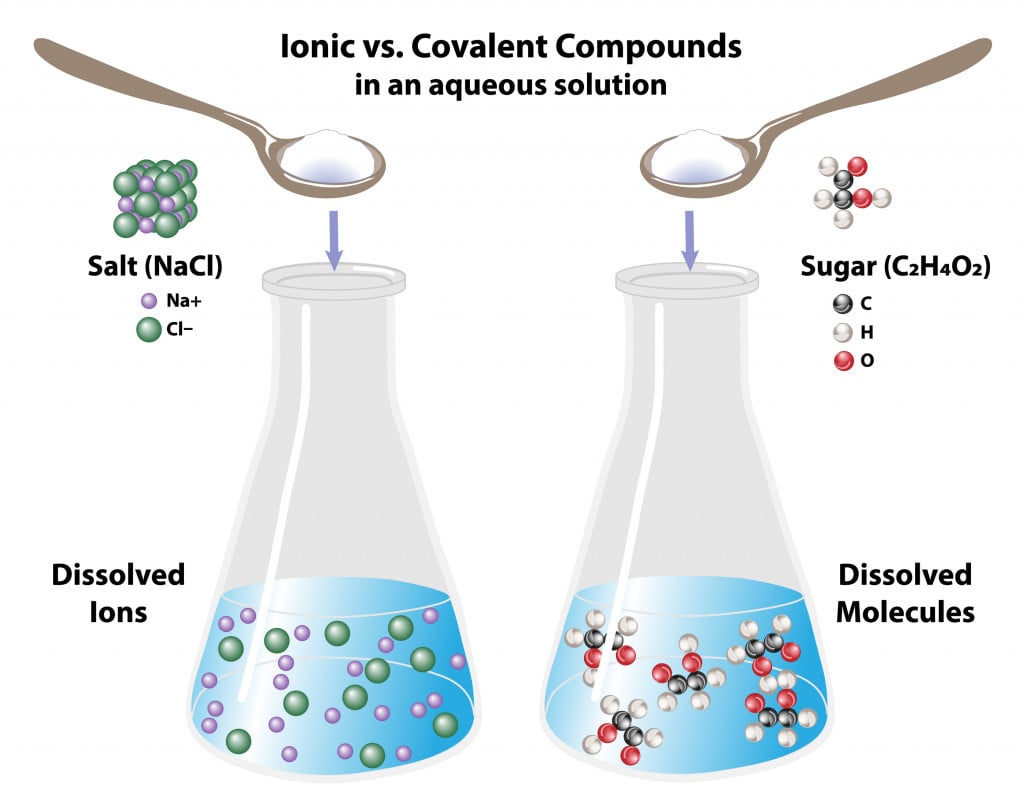
Covalent molecules like sugar remain as molecules when dissolved in water, whereas ionic molecules like salt dissociate into its respective ions.
What Is Hydrogen Bonding?
In a covalent bond, the electrons are not shared equally between the atoms. The bonding is similar to a tug of war, where the stronger one wins. Some atoms like oxygen, nitrogen, and fluorine are highly electronegative, which means they have the power to pull the electrons closer to them. As a result, in the bond, one end will be more negative than the other.
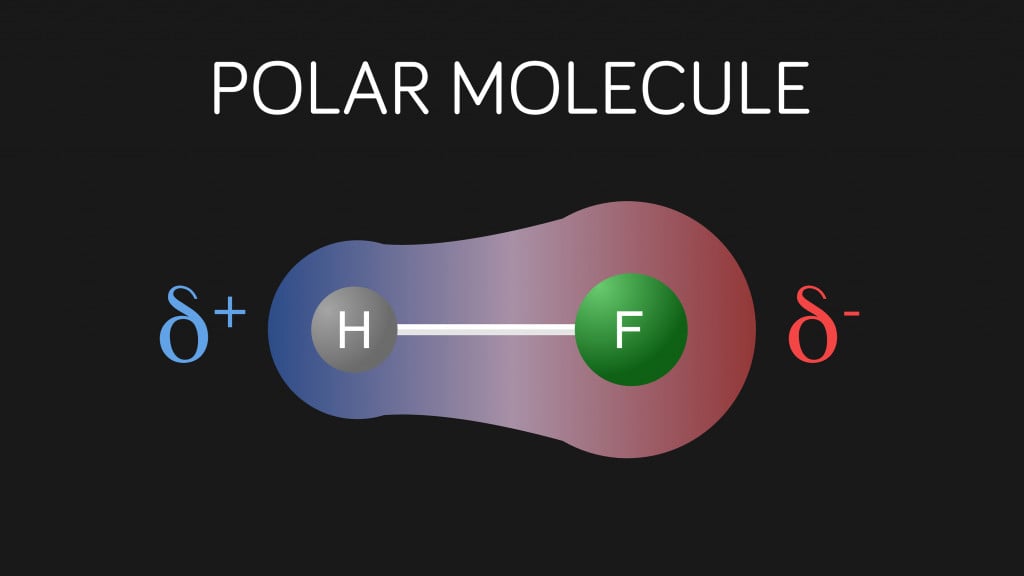
Oxygen has an electronegativity of 3.44, whereas that of hydrogen is 2.20. Hence, oxygen exerts a stronger pull on the electron pair. Thus, in an O-H bond, oxygen has a partial negative charge and hydrogen has a partial positive charge. Partially positive H atoms of one molecule can electrostatically attract the partially negative O atoms of other molecules.
This intermolecular attraction between a hydrogen atom (with a partial positive charge) and another electronegative atom like O, N, or F (bearing a partial negative charge) is called a Hydrogen Bond. As the name suggests, it is not exactly a ‘bond’, but simply a force of attraction between polar molecules. A hydrogen bond is weaker than a covalent bond, but for an intermolecular force, it’s still pretty strong.
But what does this have to do with stickiness?
Stickiness Of Sugary Water
Water and sugar on their own aren’t sticky for two reasons.
Due to the low number of bonding atoms (2Hydrogen, 1Oxygen) and the small size of water molecules, the hydrogen bonding in liquid water is weak. These H-bonds do not hold the water molecules too tightly. As a result, the molecules can simply brush past each other in their liquid state. This is why water transfers easily to any surface and flows effortlessly.
Compared to water, sucrose is a bulky molecule. It has 8 -OH groups protruding from its carbon chain. This steric hindrance makes it difficult for the sugar molecules to come closer and have a strong hydrogen bond. Moreover, since they’re large, they cannot flow past one another with ease. Thus, they stack up to form a weak crystalline structure. This is why sugar exists as a brittle molecular crystal.
However, when water and sugar are mixed, something interesting happens. In water, the sugar molecules spread out and are free to move. Besides, it’s pretty easy for the tiny H2O molecules to get close to the OH chains of sucrose and link through hydrogen bonding. Thus, sugar and water gradually form an extensive network of hydrogen bonds. The result is a sticky, clumpy mass.
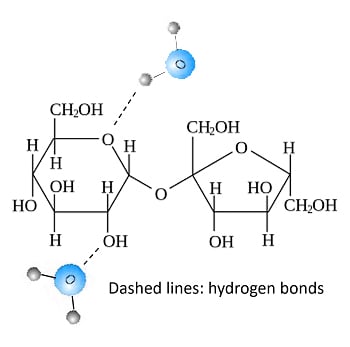
Cohesion And Adhesion
Hydrogen bonding enhances two properties that help in stickiness: cohesion and adhesion.
Cohesion is the tendency of ‘similar’ molecules to stick together. Water-water or sugar-sugar molecules in the solution stick together due to cohesion. Also, if the concentration of sugar is high, the cohesion of sugar molecules escalates due to extensive hydrogen bonding. This linkage may also result in the formation of sucrose chains. This is why sugar syrup is stringy. Cotton candies make use of this ability of sugar to form fine strings.

Adhesion is the tendency of a molecule to stick to a ‘different’ kind of molecule. Bonding between sugar and water represents adhesion. Similarly, sugar can also adhere to other polar molecules. For example, our skin is a polar tissue and sugar is also polar, so they can ‘stick’ together. Adhesion is the reason why sugar solutions stick to our hands and utensils.
The ratio of cohesive and adhesive forces determines the overall ‘stickiness’ of a substance.
Increased cohesion and adhesion imparts some resistance to the flow of a solution. This resistance of a fluid, called viscosity, is responsible for the thick, viscous nature of sugar syrup or honey.
Conclusion
Now we know why sugar solutions make such a sticky sweet mess. The extensive hydrogen bonding between sugar and water molecules improves the cohesive and adhesive properties of the system, thereby increasing its stickiness. Now you understand the not-so-simple chemistry behind sticky sugary solutions!
References (click to expand)
- Burke, J., & Hartel, R. W. (2021, February). Stickiness of sugar syrups with and without particles. Journal of Food Engineering. Elsevier BV.
- Imberti, S., McLain, S. E., Rhys, N. H., Bruni, F., & Ricci, M. A. (2019, December 18). Role of Water in Sucrose, Lactose, and Sucralose Taste: The Sweeter, The Wetter?. ACS Omega. American Chemical Society (ACS).
- Chemical Bonds. Georgia State University
- Periodic Trends — Electronegativity. Angelo State University
- 7.3: Hydrogen-Bonding and Water - Chemistry LibreTexts. LibreTexts
- Crystallography. The structure of crystals. The Spanish National Research Council













