Table of Contents (click to expand)
Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. The level of humidity in the air also plays a role in the process of evaporation.
In school, we were taught that water changes from liquid to vapor when it boils, which requires a high temperature called the boiling point. For water, this point is 100°C.
But we have all seen that puddles evaporate when the skies clear after rain, even when the temperature is not near 100°C. This is because water can also change from liquid to vapor during evaporation, which happens at much lower temperatures.
The reason for this lies in the physical and chemical properties of water molecules and the bonds they form with each other, known as intermolecular bonds.
Recommended Video for you:
Water And Its Covalent Bonding
A water molecule is composed of two hydrogen atoms attached to one Oxygen atom. The sharing of electrons forms the bonds between the O and H atoms. These bonds are called covalent bonds. Every element tends to attain the energetically lowest state (i.e., the most stable state) by losing or gaining electrons to reach the nearest noble gas configuration.
Imagine a water molecule as a family, where oxygen is the parent, and the two hydrogen atoms are the children. The bond that holds the family together is like the bond between oxygen and the hydrogens – we call those bonds covalent bonds because the atoms share their electrons, much like siblings sharing toys.
Now, let’s talk about why these elements decided to bond in the first place.
Each element wants to be in its happiest and most relaxed state. You can think of this as trying to get the perfect sleep at night. Not too little, not too much, but just right.
For elements, this perfect state is called the noble gas configuration, which is like their ultimate goal in life. They either lose or gain electrons to reach this goal, much like we lose unnecessary stress or gain more rest to reach our perfect rest.
Water is a molecule that consists of one Oxygen atom and two Hydrogen atoms. Oxygen requires two electrons to complete its outermost shell, while Hydrogen needs one. By sharing electrons, Oxygen and Hydrogen atoms can form a molecule of water, i.e., H2O.
Oxygen’s high electronegativity enables it to attract electrons towards itself, forming a partial negative charge on the Oxygen atom. Similarly, a partial positive charge develops on the Hydrogen atoms.
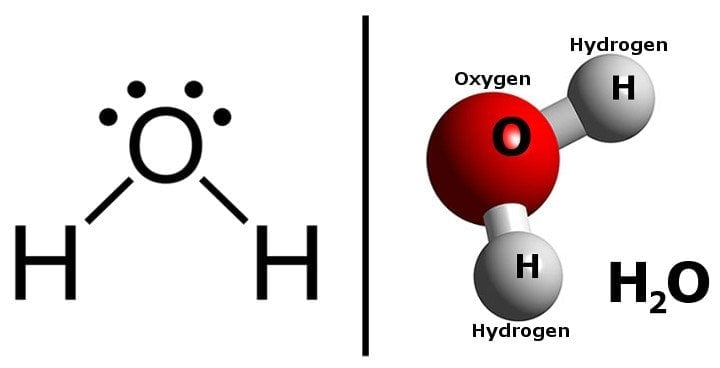
Due to the molecule’s geometry, positive and negative charges are separated between the Hydrogen and Oxygen atoms.
When two water molecules are nearby, a weak Hydrogen Bond bond can form between the partially negative Oxygen atom of one molecule and the partially positive Hydrogen atom of the other. This bond exists between two different molecules and is classified as an intermolecular bond. Hydrogen bonds are weak, requiring less energy to break, which is why water remains a liquid at room temperature.
Temperature And Molecular Energy
Temperature is a measure of the average kinetic energy possessed by a molecule. The higher the temperature, the greater the average energy, and the easier it is for molecules to overcome intermolecular attraction and move more freely. For a liquid to change phase to vapor, two forces must be overcome.
The first is the intermolecular attraction from nearby molecules, called cohesive forces. The second is the downward pressure exerted by the atmosphere. When a liquid changes phase to vapor, its molecules have acquired sufficient kinetic energy to overcome all the intermolecular forces and also overcome the downward pressure exerted by the atmosphere around it.
Humidity
The amount of water vapor present in the atmosphere is called humidity. The atmosphere can hold only a fixed amount of water vapor at any given temperature. The greater the temperature, the greater the amount of water vapor in the atmosphere. The concentration of water vapor in the atmosphere has an upper limit beyond which no water vapor can be held.
Evaporation At Room Temperature
Assume that water is spread thinly on a table.
The topmost layer of molecules experiences attractive intermolecular forces only from the bottom and sides, while those within the bulk of the liquid experience intermolecular attraction from all directions. This means that the top layer experiences less net intermolecular forces than those within the bulk.
Weak intermolecular forces (Hydrogen Bonds) allow some molecules at the top layer to gain sufficient kinetic energy to escape into the atmosphere, even at room temperature, when exposed to sunlight.
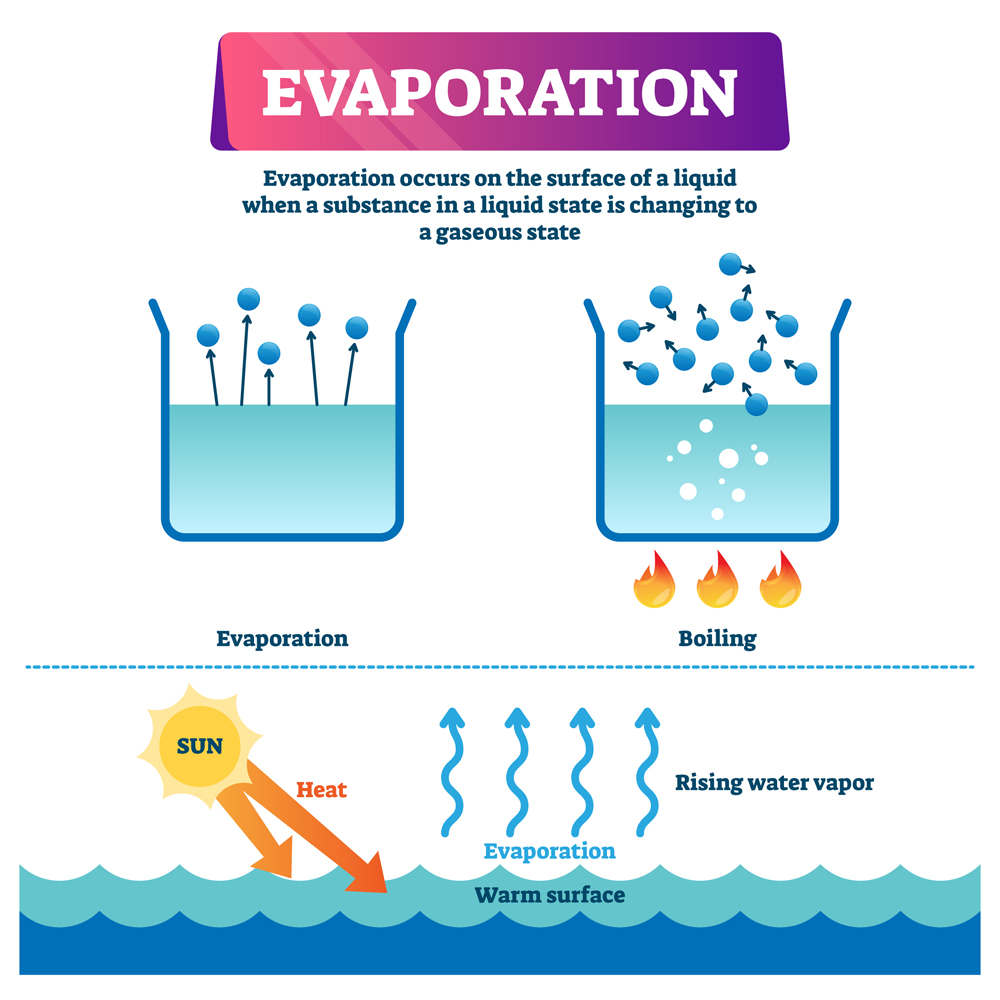
Lower humidity makes it easier for the liquid to evaporate. As evaporation continues, the concentration of water vapor in the atmosphere increases. When the atmosphere reaches a critical threshold, no more water vapor can be held and becomes saturated. Evaporation continues if the saturation state hasn’t been reached.
Therefore, a combination of humidity and high molecular energy allows some molecules at the surface to evaporate, even at lower temperatures.
Last Updated By: Ashish Tiwari
References (click to expand)
- Macleod, D. B. (1925). The kinetic theory of evaporation. Transactions of the Faraday Society. Royal Society of Chemistry (RSC).
- Allen, L. C. (1994, January 20). Chemistry and electronegativity. International Journal of Quantum Chemistry. Wiley.
- Saturated Vapor Pressure.
- 7.3: Hydrogen-Bonding and Water.
- electronegativity.
- Kinetic Temperature, Thermal Energy.












