Tap water contains atmospheric gases, such as nitrogen and oxygen, dissolved in it. As the glass filled with water sits out for a few hours, its temperature rises slightly (water gets warmer), which causes the dissolved gases in it to come out of the water and form bubbles along the inside of the glass.
Fill a glass with water (at or below room temperature) and leave it undisturbed for a few hours (you can do this using tap water). You will eventually notice that tiny bubbles appear along the side of the glass (on the inside).
Why does this happen?

Recommended Video for you:
Solubility Of Gases In Water
The water we use daily comes from taps, passing through pipes before reaching large storage tanks. Due to the pressure in the pipes and the coolness of the water, certain gases like nitrogen and oxygen, which are abundant in our atmosphere, can be dissolved in the water.
Gases Are More Soluble In Water At Colder Temperatures
Gases are generally more soluble in water at a lower temperature. In other words, the solubility of gases in water decreases as the water temperature rises.
The graph below should help to visualize how the solubility of gases varies with changing temperature.
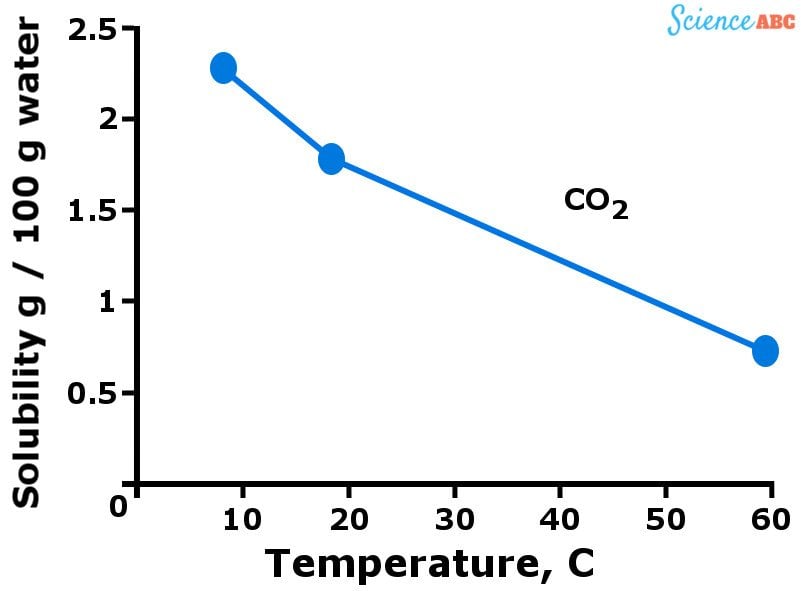
The downward trend of gases’ solubility with increasing temperature is quite similar to how vapor pressure increases with temperature. You see, more gas molecules are dissolved in water when it’s cold. However, when water starts getting warmer (i.e., the temperature rises), the kinetic energy of gas molecules also increases.
This allows those molecules to move more freely and break the intermolecular bonds that hold them together, thus escaping from the solution.
That’s why the solubility of gases decreases as temperature rises.
Danger To Aquatic Life Due To Changing Water Temperatures
The solubility of gases in water is influenced by temperature, which consequently has a direct impact on the lives of aquatic organisms. Fish and other aquatic life forms require oxygen for survival, which they obtain by absorbing the dissolved oxygen in the water through their gills. As the solubility of oxygen in water is higher in colder temperatures, it is crucial to maintain the water temperature below a certain limit.

However, the current scenario is that water temperature is increasing beyond the limit, primarily due to human activities.
For instance, power plants discharge large amounts of hot water into water bodies, leading to a rise in water temperature and adversely affecting aquatic life. This is a highly undesirable outcome of the relationship between gas solubility and temperature.
The Solubility Of Gases In Water Increases With Increasing Pressure
Although liquids and solids exhibit practically no solubility change with changing water pressure, gases do. It has been observed that gases are more soluble in water at higher pressures. Carbonated drinks are excellent examples of this phenomenon.
Henry’s Law
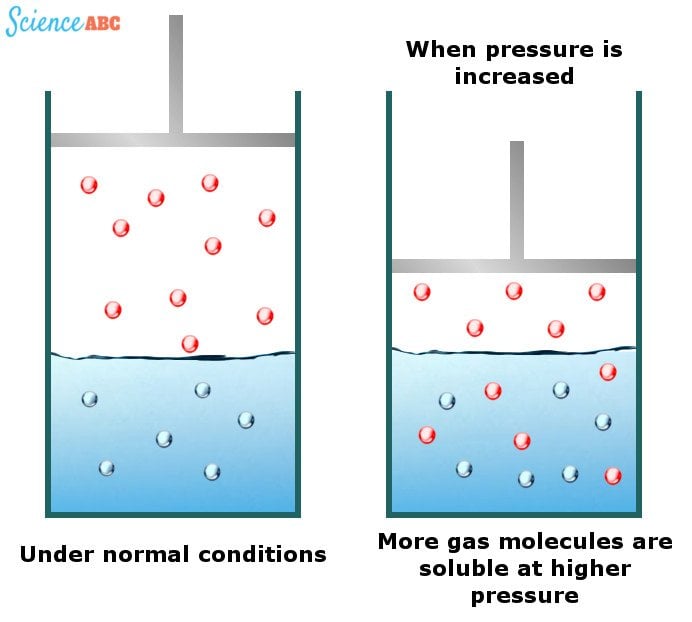
The effects of changing water pressure on the solubility of gases can be explained by one of the gas laws known as Henry’s law. It states that “the amount of dissolved gas is proportional to its partial pressure in the gas phase.”
The simplest way to explain this dependence is that gas molecules are forced into the solution as pressure increases to relieve the applied pressure. The following image should help to visualize this better:
Why Bubbles Form In A Glass Full Of Water Overnight
Due to the two physical phenomena described above, tap water becomes a good candidate for holding dissolved atmospheric gases, if not ideal. However, when this water is poured into a glass and left at room temperature for a few hours, the atmospheric pressure begins to drop as its temperature rises.

As a result, the dissolved gases in the water escape from the solution and form bubbles in the rough areas inside the glass. As the temperature does not change so quickly, it takes a few hours for the bubbles to appear on the glass.
Last Updated By: Ashish Tiwari
References (click to expand)
- Battino, R., & Clever, H. L. (1966, August 1). The Solubility of Gases in Liquids. Chemical Reviews. American Chemical Society (ACS).
- https://antoine.frostburg.edu/chem/senese/101/solutions/faq/temperature-gas-solubility.shtml
- Laws of Gas Transport.
- Hildebrand, J. H. (1916, August). Solubility. Journal of the American Chemical Society. American Chemical Society (ACS).













