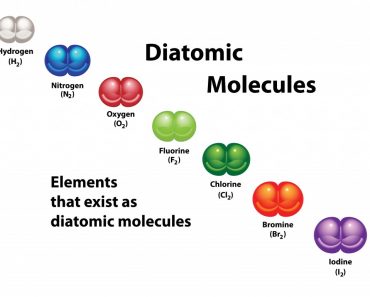
The octet rule states that atoms tend to prefer having 8 electrons in their valence shell in order to possess an electronic configuration similar to the closest noble gas.
The octet rule is one of the chemical “rules of thumb” stating that atoms prefer to combine in a manner such that each atom has 8 electrons in their valence shells. A valence shell is the outer shell of an atom. Eight electrons in the outer shell allows atoms to have a configuration that is similar to the closest noble gas. This, in turn, makes them more stable.
In order to have 8 electrons in their valence shell, atoms tend to form covalent or ionic bonds. Ionic bonding is seen in sodium chloride or salt, while covalent bonding is seen in carbon dioxide or CO2. The bonds are formed by the atoms in order to be less reactive and enjoy more stability.
To understand how and why atoms require 8 electrons in the valence shell to be in a stable state, let’s begin by understanding the way electrons are distributed in an atom.
Recommended Video for you:
How Are Electrons Distributed In An Atom?
An atom is the smallest particle of an element, yet within it, there is a universe of its own. The 3 subatomic particles that form a part of this tiny universe are neutrons, protons and electrons. The neutrons have no net charge and are in the center of the atom, along with positively charged protons. The neutrons and the protons together form the nucleus of the atom. The electrons are negatively charged particles that circle the nucleus like planets around the sun.
Electrons and protons are attracted to each other by an electromagnetic force—a physical interaction between electrically charged particles. Therefore, separating electrons from the nucleus requires a great deal of energy. The closer the electron is to the nucleus, the higher the energy needed to separate it.
Electrons revolve in different shells named K, L, M and N, beginning from the nucleus and expanding outwards. Each progressive shell has a different energy level, corresponding to 1, 2, 3 or 4. The maximum number of electrons that each shell can contain is given as 2(n2) where n corresponds to the energy level. Thus, K has 2 electrons [2(1)2= 2], L has 8 electrons, M has 18 electrons and N has 32 electrons.
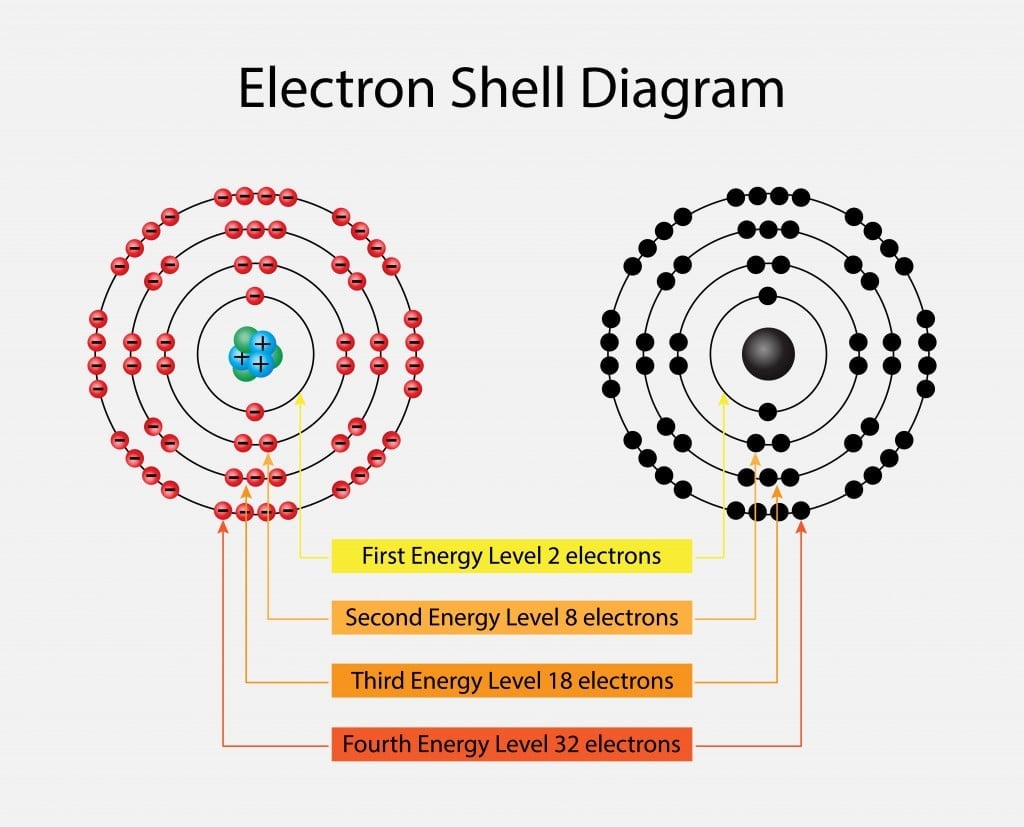
Furthermore, these shells are divided into sub-shells containing atomic orbitals. The shells contain orbitals named s, p, d, and f, starting from the nucleus. The arrangements of electrons in shells and sub-shells is called the electronic configuration of an atom.
The number of shells increases depending on the number of electrons present in the atom. The outermost shell in the element is called the valence shell and the electrons found there are called valence electrons.
What Are Valence Shells?
Valence electrons contribute to an atom’s tendency to take part in chemical reactions. An atom with a closed shell, containing 8 electrons in its valence shell, is considered to be chemically inert, as it hardly participates in chemical reactions. The atoms of such elements will then have an electronic configuration similar to that of noble gases.
The periodic table is a tabular arrangement containing all known elements arranged by their electronic configuration, nature and atomic number. At the extreme right end of the table is the group of elements called noble gases. They are considered to be stable, as they have a completely filled valence shell. The 8 noble gases are Helium (2), Neon (10), Argon (18), Krypton (36), Xenon (54) and the radioactive element Radon (86). Each bracket contains the atomic number of the atom.
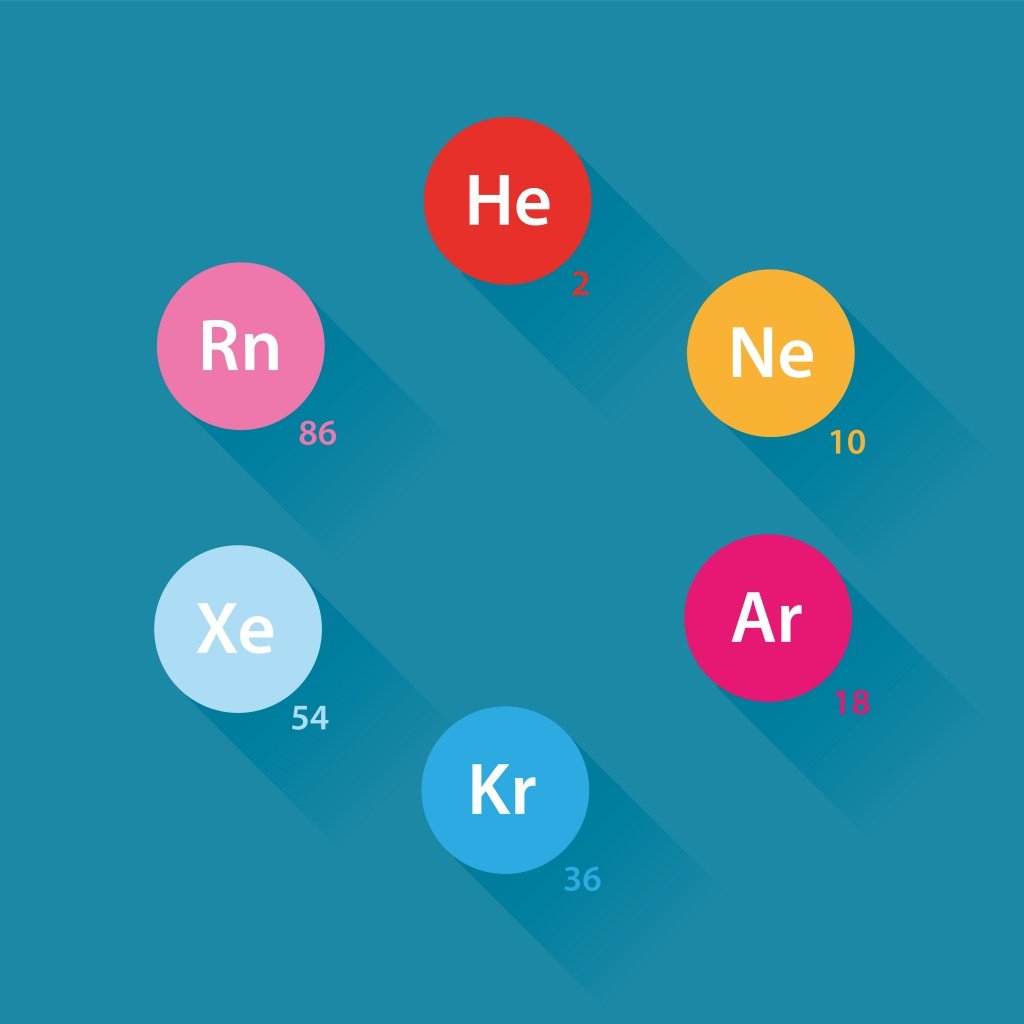
Noble gases are the least reactive because their outer energy levels are already full. The presence of 8 electrons in their outer shell makes the valency of noble gases zero. Other reactive atoms tend to fill their outer shell with 8 electrons by following the octet rule in order to approach the stability of a noble gas.
The octet rule only considers those electrons that are present in the s and p sub-shells. These sub-shells can hold up to 8 electrons in their outermost shell.
You may consider valence electrons to be some of the most important electrons that an atom possesses, as they are the electrons situated in the highest energy level. These are the electrons that most commonly get involved in chemical reactions. Valence electrons can be counted using the Lewis electron dot structure.
What Is The Lewis Electron Dot Structure (LEDS)?
The Lewis structure is named after Gilbert N. Lewis, who first introduced the concept in his article, “The Atom and the Molecule” in 1916. Lewis structures only represent the valence electrons, which are identified as dots around the atom, each dot representing one valence electron. Most of the time, atoms with less than 8 electrons in their valence shell prefer to form compounds by either covalent bonding or ionic bonding.
The formation of such bonds can satisfy the octet rule. The elements in the second group of the periodic table fulfill the criteria for 8 electrons by losing, gaining or sharing electrons between atoms.
The type of bond that is formed depends on the number of electrons in the valence shell, as well as the total energy required to form the bond. The LEDS helps us identify how many free electrons are available for a chemical reaction.
What Is A Covalent Bond?
A covalent bond is a linkage between 2 atoms that allows for sharing electrons between them, such that each atom has an electronic structure similar to that of the closest noble gas.
Take carbon dioxide, for example: carbon and oxygen have atomic numbers of 6 and 8, respectively. Carbon has 2 shells, K and L, with 2 electrons in the K shell, leaving 4 electrons in the L shell. To attain stability, carbon can either lose all 4 electrons and be closest to helium, or gain 4 electrons and be closest to neon. The energy required to gain or lose 4 electrons is immense, due to the attractive force between protons and electrons in the nucleus. However, the middle ground option is to share its electrons.
Similarly, oxygen has 2 electrons in the K shell and 6 electrons in the L shell. It only needs 2 electrons to be stable and attain an electronic configuration similar to neon. Gaining 2 electrons is easier than losing 4 electrons (which would make it similar to helium). As an adjustment, 2 oxygen atoms prefer to share their electrons with a single carbon atom so that all three have 8 electrons in their valence shell and are each individually stable.
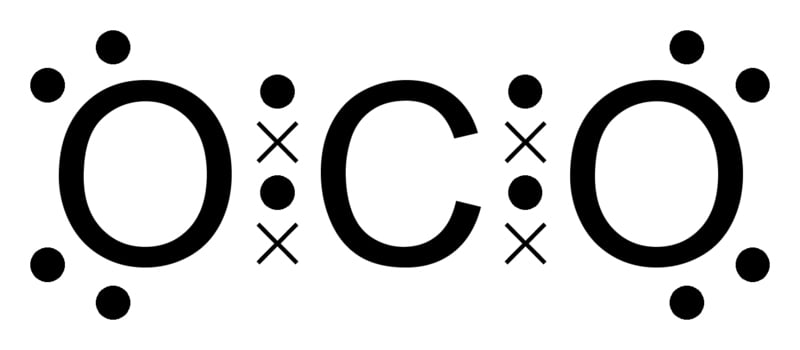
The result is a molecule of carbon dioxide with one carbon atom and 2 oxygen atoms, each individually having 8 electrons in their valence shell.
What Is An Ionic Bond?
An ionic bond is a type of chemical bond that occurs between oppositely charged ions. An atom can either be positively or negatively charged, depending on the number of protons or electrons the atom has in excess. An ion is an atom with a net charge due to the gain or loss of electrons. Keeping in mind that electrons are negatively charged, if an atom gains an electron, it will have a negative charge, but if it loses an electron, it will have a positive charge. In short, an ion is an atom that contains a particular charge.
An ionic bond occurs between metals and non-metals, where one is electropositive and the other is electronegative. The electropositive metals lose an electron to become a positive ion, called a cation, whereas the electronegative nonmetals accept an electron and become a negatively charged ion, called an anion. The loss and acceptance of electrons is the same in order to form an ionic bond, and at the same time, each atom satisfies the octet rule!
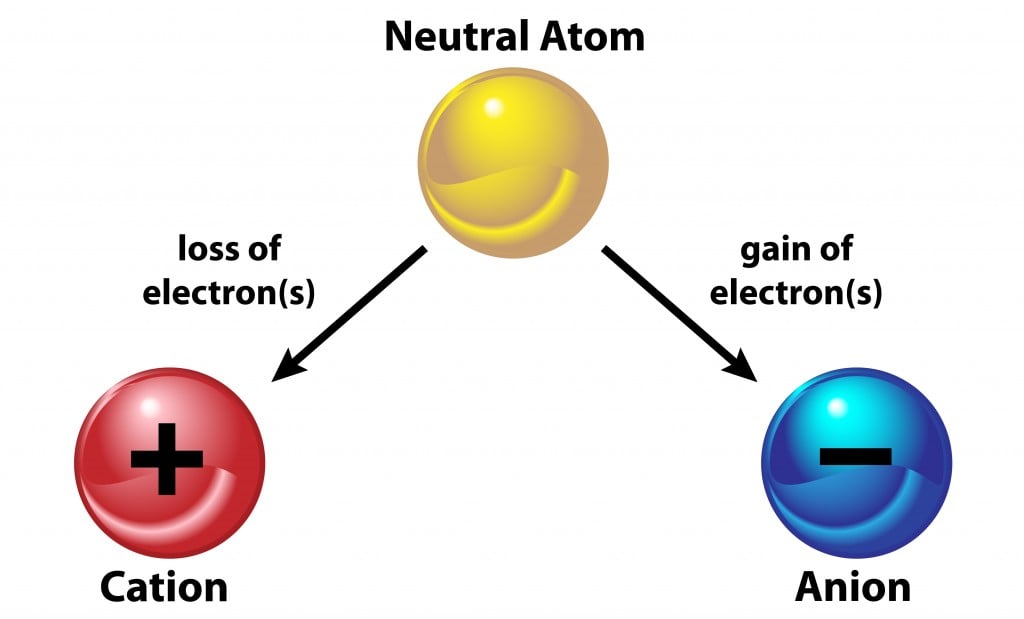
So, when an atom has an unequal number of electrons and protons, it is called an ion. As a result, 2 atoms with unequal charges come together by either losing or gaining their electrons, thus attaining a neutral charge through an ionic bond. They remain bonded with the help of the attractive forces between them. The net result is that each atom is stable, as it has a completely filled valence shell with 8 electrons.
Salt or sodium chloride is a great example of an ionic bond. Sodium has an atomic number of 11 with 2, 8, and 1 electrons in the K, L and M shells, respectively. This leaves 1 electron in the valence shell, which the sodium atom easily loses during a reaction. Chlorine has an atomic number of 17 with 2, 8, and 7 electrons in the K, L and M shells. Chlorine has 7 electrons in its valence shell, making it highly electronegative. Each atom just needs to adjust 1 electron to have a completely filled valence shell and attain a configuration similar to the closest noble gas.
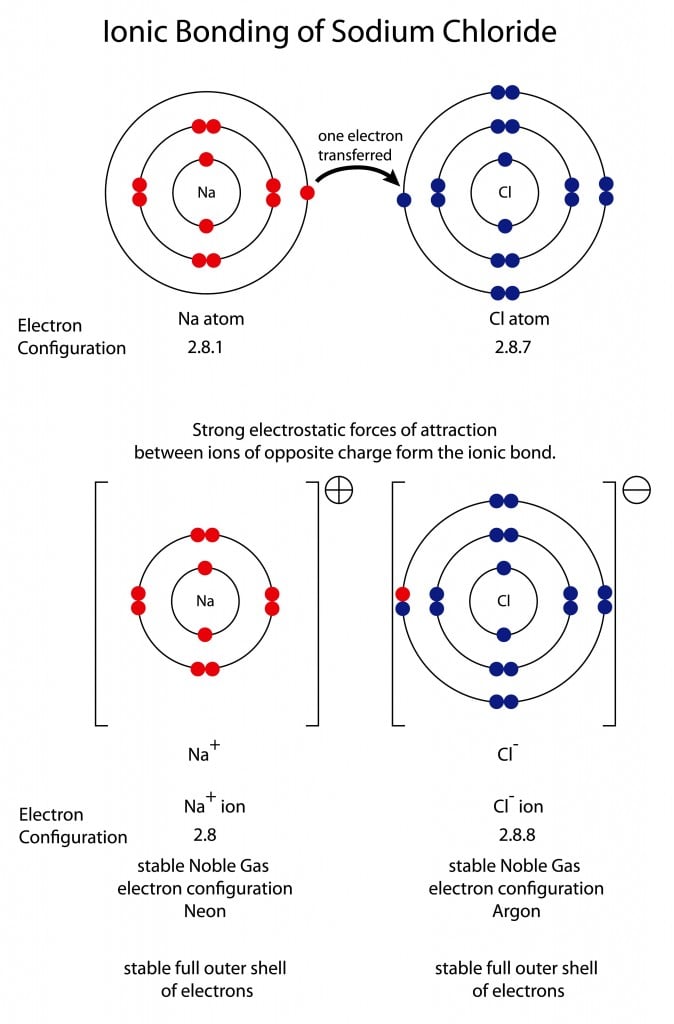
The energy required to either gain or lose 7 electrons, in the case of sodium or chlorine, is tremendous. To make the process easier, sodium loses its lone electron and acquires an electron configuration similar to neon (10). Chlorine accepts the electron lost by sodium and achieves an electron configuration similar to argon (18). The end result is an ionic bond between sodium and chlorine, giving us salt.
I bet you never thought that basic table salt could have such an intricate chemical history!
What Are The Exceptions To The Octet Rule?
There’s always that one kid in class who loves breaking the rules and the same thing is true in chemistry… there are some examples of elements that don’t follow the octet rule. Let’s take a brief look at them.
Molecules with an odd number of electrons generally don’t follow the octet rule. Also, atoms with only 2 electrons (in total) fall under this category.
Hydrogen atoms, with an atomic number of 1, can hold a maximum of 2 electrons, but these atoms only have one electron shell with an s orbital. As the octet rule applies to the s and p orbitals only, atoms with 2 electrons are exceptions to the rule.
Lastly, molecules in which one or more atoms have more or less than 8 electrons are also exceptions.
The octet rule is one of the most important rules in chemistry and governs how atoms react with each other to hold 8 electrons in their valence shell. Forming covalent or ionic bonds is the most convenient way for atoms to satisfy the octet rule. After all, atoms probably get tired of being reactive all the time and follow the octet rule just to have some peace and stability!
References (click to expand)
- Walton, J. R., Rivera-Rivera, L. A., Lucchese, R. R., & Bevan, J. W. (2017). Is there any fundamental difference between ionic, covalent, and others types of bond? A canonical perspective on the question. Physical Chemistry Chemical Physics. Royal Society of Chemistry (RSC).
- What is an ion?.
- Ionic Bonding - an overview | ScienceDirect Topics.
- http://ruby.colorado.edu/~smyth/G101-2.html