Table of Contents (click to expand)
A phosphodiester bond is formed between two sugar molecules and a phosphate group. This bond connects nucleotides, which form the backbone of a DNA or RNA chain.
DNA and RNA, as we know, are extremely important biomolecules found in living organisms. They are responsible for making us what we are—similar, and yet so unique. Every person is aware of the famous double helix structure of DNA. If nothing, then you have seen it in movies like Spiderman: a winding, ladder-like structure, like a spiral staircase. Phosphodiester bonds are bonds between the phosphate group and the 2 sugar molecules in DNA or RNA.
Recommended Video for you:
Structure Of DNA And RNA
To understand a phosphodiester bond, we first need to understand the basic structure of DNA and RNA. We know that DNA has a double helix structure, whereas RNA has a similar structure, except that it only has a single strand.
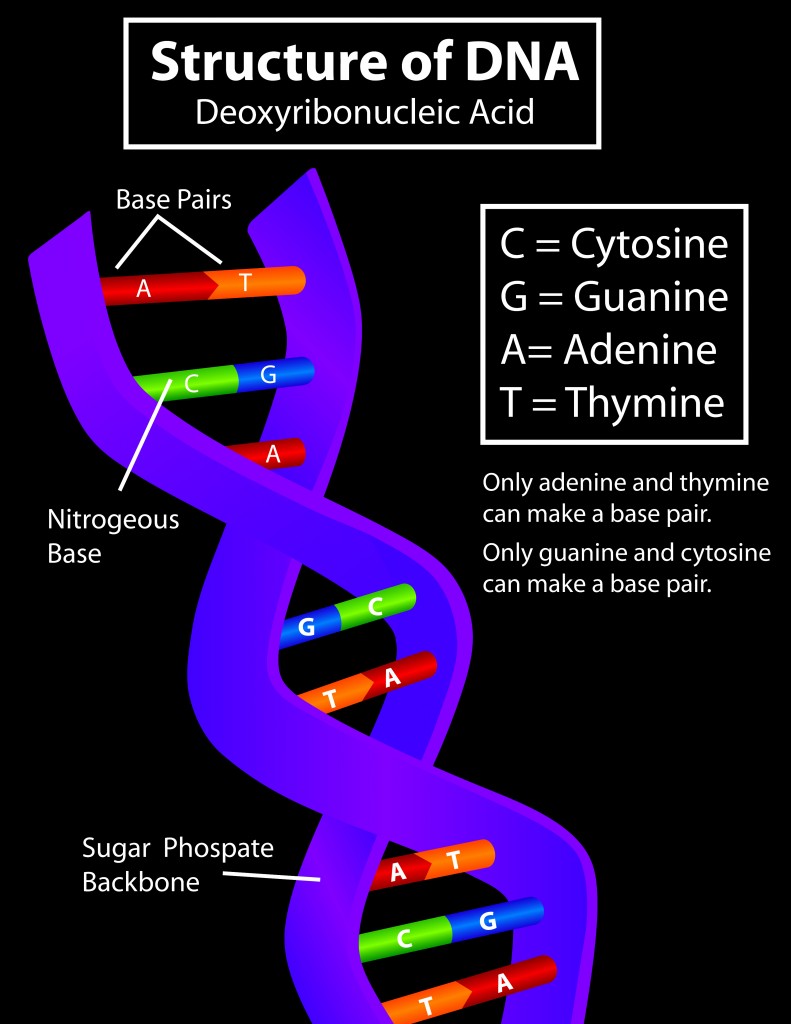
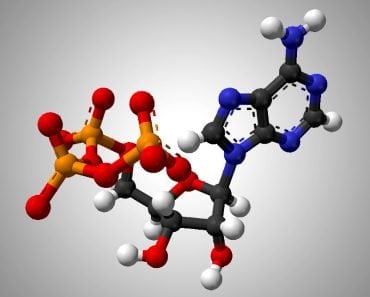
DNA consists of three parts–a nitrogenous base, a sugar molecule and a triphosphate group. There are four nitrogenous bases: adenine, guanine, cytosine and thymine (uracil in RNA). The base, attached to the sugar molecule, is known as a nucleoside. The nucleoside attached to the phosphate group is called a nucleotide.
Nucleotide
As mentioned above, a nucleotide molecule consists of 2 parts–a nucleoside and a phosphate group. Interlinked nucleotides form a single strand of genetic material. In the case of DNA, two strands are linked together by their nitrogenous bases to form the double-stranded structure.
The sugar molecule in RNA is a ribose sugar, which is a 5-carbon sugar molecule (C5H10O5). In DNA, the sugar molecule has one oxygen atom less, which is why it’s called deoxyribose (C5H10O4). This sugar molecule is linked to the phosphate group. The phosphate group comes from phosphoric acid (H3PO4), which has lost 2 hydrogen atoms.
The attachment occurs at the 5th carbon of the sugar molecule. The carbon atom has 2 hydrogen atoms, and a hydroxyl group (-OH group). During bond formation, the phosphate group loses a hydrogen atom, while the 5th carbon of the sugar loses a hydroxyl group. Thus, a bond is formed between them with a water molecule, formed by the H of the phosphate group, and the OH of the sugar is released. This is an ester bond.
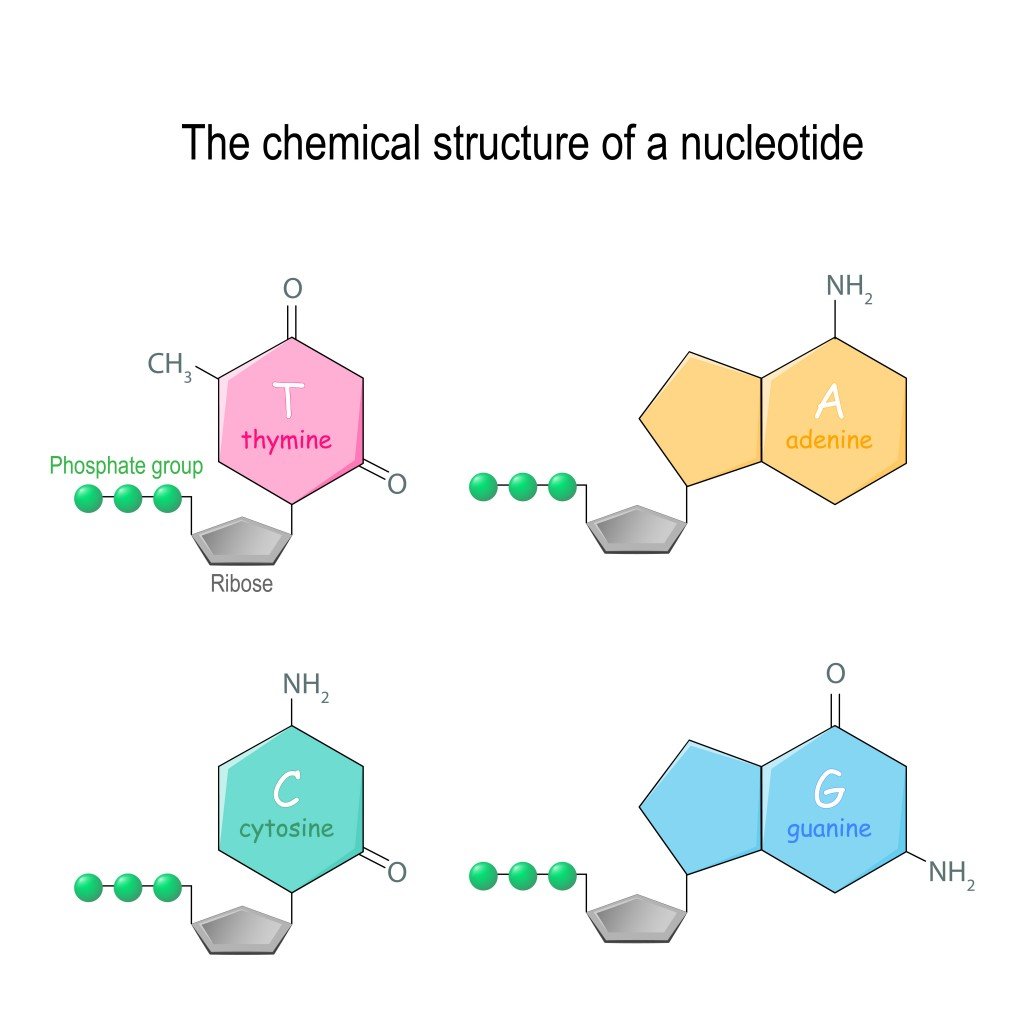
It is important to note here that nucleotides can have one, two or three phosphate group at the 5th carbon. However, in nucleic acids, there are three phosphate groups.
Phosphodiester Bond
A phosphodiester bond literally refers to the time when a phosphoric acid molecule forms two ester bonds. As shown above, a nucleotide molecule already has one ester bond when the nucleoside attaches to the phosphate group. This phosphate group attaches with the sugar molecule of the neighboring nucleotide to link them. This sugar-phosphate-sugar bonding forms the backbone of the DNA or RNA strand.
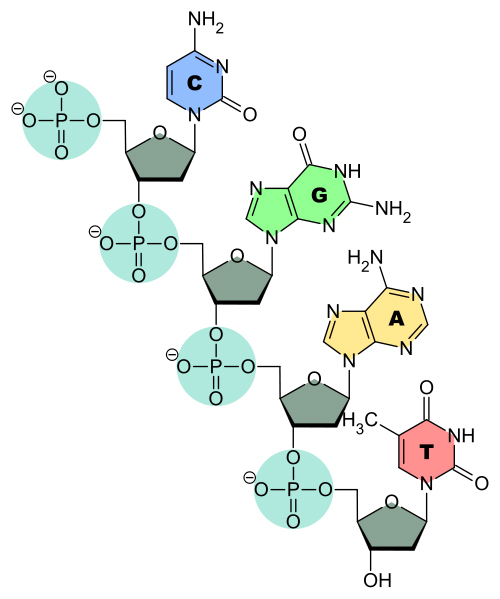
Consider a phosphoric acid molecule. It has already lost one hydrogen atom to a sugar molecule to form a nucleotide. This phosphoric acid now undergoes a similar process to link with another sugar molecule. However, the difference is that when it links to the sugar molecule while forming the nucleotide, it attaches at the 5th carbon of the ribose sugar. During bond formation between 2 different nucleotides, the hydroxyl group (-OH) is lost from the 3rd carbon of the ribose sugar. The same process ensues—a hydrogen is lost from the phosphoric acid and an -OH is lost from the sugar to form a water molecule.
This forms the second ester bond, so it gets the name phosphodiester bond.
As mentioned, phosphodiester bonds can be in nucleotides containing a monophosphate, diphosphate or triphosphate. In DNA and RNA, however, the nucleotides have a triphosphate group.