Table of Contents (click to expand)
Buckyballs or fullerenes are a class of allotrope of the element carbon, and are infamous for the determination of their structure. They commonly compose the black, smooth soot commonly found in furnaces and fireplaces.
Though this may seem like an article about Marvel’s Bucky Barnes, it isn’t. It is about something even more interesting… from the world of chemistry! Buckyballs, fullerenes or Buckminsterfullerenes are the various names given to a group of fairly recently discovered forms of elemental carbon.
Recommended Video for you:
What Are Buckyballs Or Fullerenes?
This may be hard to believe, but fullerenes are molecules consisting of carbon atoms in large numbers. Usually, they consist of 60 carbon atoms that form one molecule of the “buckyball”. Sometimes, however, their structure might also be 70, 76, 84, 90, 94, or even more carbon atoms.
They are spherical in shape, so much so that together with their symmetry and sphere-like shape, they closely resemble a soccer ball. This structure is called a truncated icosahedron, in technical terms. It is a polygon with 60 vertices and 32 faces. 12 of these faces are pentagonal, while 20 are hexagonal.
This spherical shape and the interconnections between the carbon atoms create a huge cage-like structure to this allotrope of carbon. This structure was initially difficult to determine. Who would have ever thought that a 60-carbon molecule would turn out to resemble something as simple as a football?
You might guess that the name “Buckminsterfullerene” has been given as a tribute to the scientist who discovered it, but that’s absolutely not the case.

Buckminster Fuller is the name of an architect renowned for his geodesic domes. It was these domes that gave an idea to scientists about the structure of fullerenes. As a tribute to his inspiration, this group of compounds was named after him.
Discovery Of Fullerenes
Fullerenes were discovered accidentally, like so many great realizations in the world of science. A group of scientists was researching certain particles found in space when they came across this special group of molecules.
Fullerenes were discovered in 1985 by British chemist Harold W. Kroto of the University of Sussex, along with Rice University chemists Robert F. Curl and Richard E. Smalley. The team of researchers used Smalley’s experimental setup—used for things as small as a rice grain—and began their experiments. They needed this, since the scientists were dealing with atoms and molecules even smaller than a rice grain.

Carbon atoms were vaporized from graphite (another allotrope of carbon) and the technique of mass spectroscopy was used for further inspection. Through this, it was concluded that the vaporized carbon atoms came together to form a 60-carbon molecule.
There were other clusters of a different number of carbon atoms, but they broke off, making the mass spectroscopy signals vanish for them after some time.
This is how the idea of a 60-carbon atom allotrope existing came into existence!
Chemical Properties Of Fullerenes
To identify the structure of fullerene as a soccer ball was a tremendous task, because this allotrope of carbon was ridiculously unreactive! It made the scientists and researchers scratch their heads as to what could make this huge molecule so hugely inert.
As it turns out, fullerenes tend to be inert because of their closed cage-like structure. Chemically, these groups of molecules are this way because they do not have any ‘dangling bonds’ or loose bonds. A loose bond is just a free bond that makes a molecule react with other species.
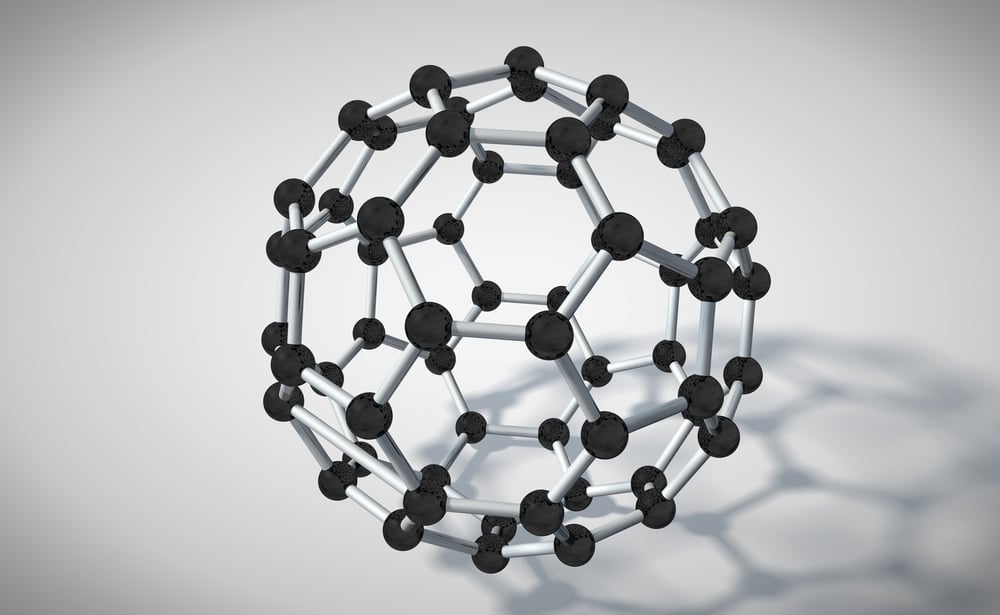
Each carbon is connected to the others by two single bonds and one double bond; the number of bonds a carbon atom can form is four. Buckyballs are soluble in organic solvents like benzene, so shaking up the soot (composed of fullerenes) with toluene results in a red-colored solution!
Buckminsterfullerenes behave as electron-deficient species, and therefore prefer to react with species that are rich in electrons, for example, the halogens or the hydroxyl group (-OH group). When they react with -OH, they are made into ‘fullerenols’.
Fullerenes tend to form two types of compounds—exohedral and endohedral. The former category comprises reacting species being present outside the cage, while the latter represents the inside the cage situation.
Exohedral species are synthesized due to a chemical reaction, mostly an addition or a redox reaction, between the fullerenes and other reactive groups, such as the –OH group mentioned above.
Endohedral species, on the other hand, are synthesized when the transition metal complexes or metals like Lanthanum or Iridium get trapped within the cage.
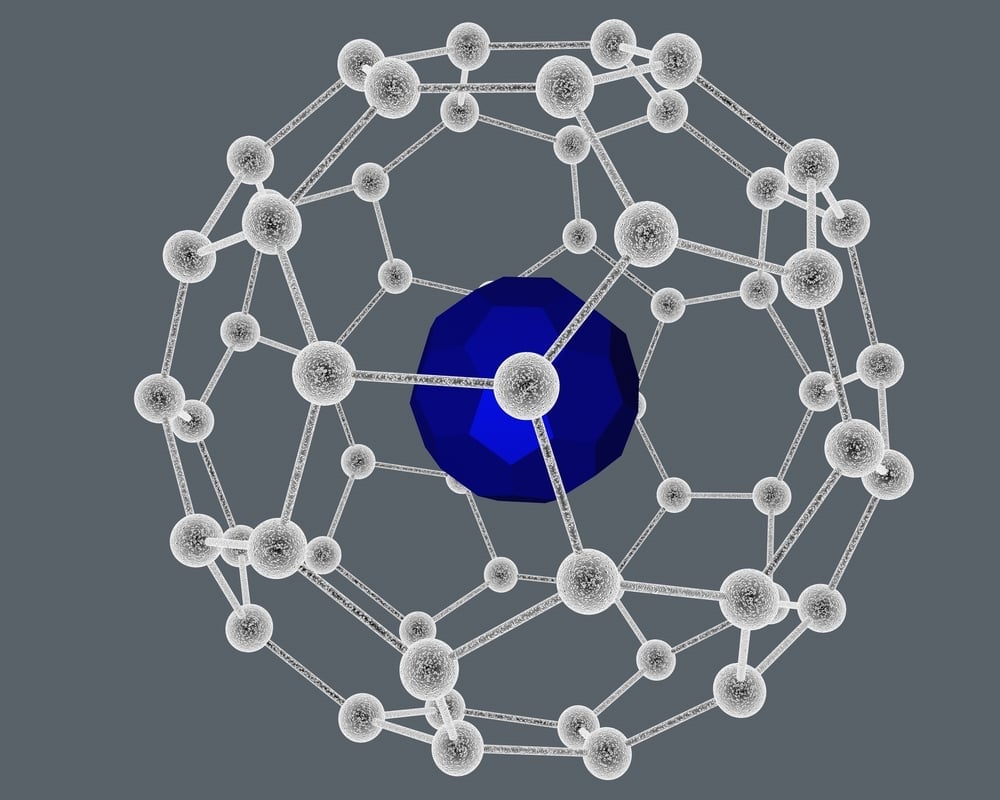
Even though fullerenes are themselves mostly unreactive, they have been made to react, rather than be synthesized that way.
Applications Of Fullerenes
Due to their recent discovery and relative inertness, there have not been many clear establishments for the applications of fullerenes. However, there are predicted applications that are presently being tested.
Buckyballs are insulators by nature, but they have also been observed to possess conducting and superconducting properties under certain conditions. These properties, especially the superconducting property, would lead to immense usage in the future, especially in transistors and microchips!

There are also some therapeutic uses where fullerenes could apply. Fullerenes with metal atoms caged within them are being utilized in MRI and X-rays as contrast agents. This is because these metallofullerenes are harmless to the human body. They stay in the body for about an hour, thus helping in imaging the interior of the body.
Aside from diagnostic labs, fullerenes have also been found to possess anti-HIV properties; if exploited properly, this could be one of the greatest new developments in modern medical science.
Fullerene derivatives are localized in an advantageous way, thus making them suitable for the treatment of osteoporosis. Some of the derivatives of fullerenes have also proved themselves to have anti-microbial properties.
Conclusion
Even though fullerenes have only been recently discovered and their structure has left us in awe, there are still many applications that need to be experimented on and researched further. How else will these allotropes with cute titles become practically effective for mankind!
References (click to expand)
- Discovery of Fullerenes National Historic ....
- Have buckminsterfullerenes (buckyballs) been put to any ....
- Nimibofa, A., Newton, E. A., Cyprain, A. Y., & Donbebe, W. (2018, June 30). Fullerenes: Synthesis and Applications. Journal of Materials Science Research. Canadian Center of Science and Education.
- . (1994). A Positron Named Priscilla. []. National Academies Press.
- Fullerenes: An Overview.
- Fullerenes: Chemical structure and properties.