Table of Contents (click to expand)
The Gay-Lussac’s law states, ‘At constant volume, pressure of a gas is directly proportional to its absolute temperature in kelvin’.
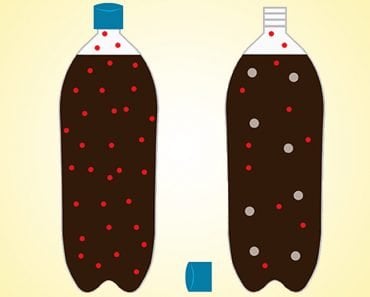
All aerosol cans come with a warning label that reads ‘Protect from sunlight and do not expose to temperatures exceeding 50°c’. When an aerosol can is exposed to high temperatures, the pressure of the gas inside it increases. After some time, a point is reached when the container can no longer hold the high-pressure gas and ultimately explodes.
The aerosol cans explode because the pressure and temperature of a gas are closely related to each other. Gay-Lussac’s law describes this relationship between the temperature and the pressure of a gas.
Recommended Video for you:
Gay Lussac Law Formula
Gay-Lussac’s law states, ‘The pressure of an ideal gas is directly proportional to its absolute temperature when the volume is held constant’. The law is mathematically expressed as,
P ∝ T
P = kT
Where P is the pressure of the gas and is usually measured in atm or mm of Hg, T is the absolute temperature of the gas in kelvin and k is the constant of proportionality.

Let’s have a look at how the temperature and pressure of a gas are defined and measured to thoroughly understand the law.
The kinetic theory of gases defines temperature as the average kinetic energy of the gas molecules. The theory assumes gases as a collection of microscopic particles (atoms in case of monoatomic gases and molecules in case of diatomic gases) that are in a constant state of motion. The motion is entirely random and proceeds at a rapid pace. The particles are also assumed to continually collide with each other and the walls of the container they are contained in.
The kinetic energy of a single molecule is given as half of the product of its mass and velocity squared (K.E = mv2/2). Since temperature is defined as the measure of the average translational kinetic energy of the molecules, temperature and motion of molecules of a gas are proportional to each other.
T ∝ mv2/2
Where T is temperature, m is the mass of a single molecule and v represents its speed of motion.
As the mass of a molecule is constant, the kinetic energy can only increase if the velocity of the molecule increases. Thus, a rise in temperature leads to an increase in the velocity of the molecules. In hot gases, the molecules move at a faster rate as compared to molecules in cold gases.
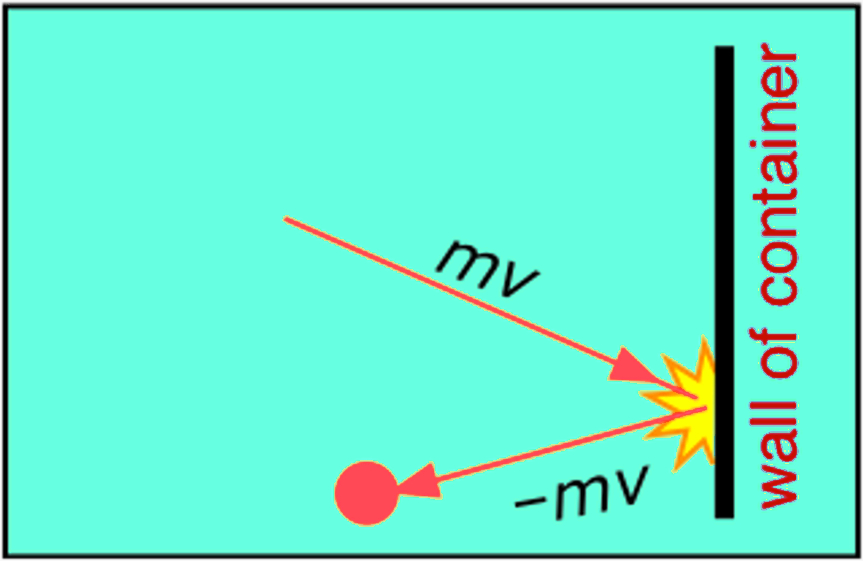
Pressure, on the other hand, is defined as the force applied/exerted per area by the gas molecules on the walls of the container. As temperature rises, so does the velocity with which the molecules strike the walls of the container. Thus, with temperature, the force applied by the gas molecules on the walls of the container and the frequency of collisions, i.e, pressure increases too.
Gay-Lussac’s law for the same gas at two different states is given as,
P1/P2 = T1/T2
P1.T2 = T1.P2
Applications Of Gay-Lussac’s Law
Gay-Lussac’s law can be seen in action in a lot of day-to-day activities. Some examples of these include the working of a pressure cooker, tire explosions in summer, in firing tools such as guns, aerosol cans, etc.
Tire explosions are a common experience during summers, in fact, tire explosions during summer are so frequent that the period between May and October is called the blowout season. The explosions occur due to the heat build-up caused because of friction between the tires and the road surface. During summers, the asphalt laid on the roads is already at a very high temperature and this adds to the dilemma.
Due to friction, the temperature of the tires (made up of rubber and thus, a good conductor of heat) increases and consequently the temperature of the air inside the tire does too. A rise in temperature of the air is followed by an increase in the pressure, as explained by Gay-Lussac’s law. The rise in pressure is also accompanied by an increase in the volume of air. Ultimately just as in aerosol cans, a point is reached when the tires can no longer hold the high-pressure air and volume of the expanding air inside and explode.
History Of Gay-Lussac’s Law
The relationship between temperature and pressure was first noticed by a French physicist, Guillaume Amontons. Although the results and conclusions Amontons drew were only partially correct. His studies and experiments with air as the sample gas lead him to conclude that the pressure of a gas increases by approximately one-third between temperatures of cold water and boiling water.
The inaccuracies were caused due to a lack of availability of accurate thermometers. Amontons also hypothesized that a sufficient reduction in temperature would cause the gas pressure to disappear entirely.
More than 100 years later, around 1802, Joseph Louis Gay-Lussac published his work on the macro properties (volume v, temperature T, pressure p, number of particles n) of gases. Gay-Lussac stumbled onto the law while developing an air thermometer.
Along with the relationship between pressure and temperature, Gay-Lussac stated another law. He stated, ‘At constant pressure, the volume of an ideal gas is directly proportional to its absolute temperature’. Although, this law is known as the Charles law; as Jacques Charles had arrived at a similar conclusion but didn’t publish it and Gay-Lussac was humble enough to give Charles the due credits.
Conclusion
Since the relationship between pressure and temperature was first discovered by Amontons, the relationship is now generally known as Amontons’ law. While Gay-Lussac’s name is associated with another law he discovered. The law is called the law of combining gases and explains how the ratio of reactant gases and their gaseous products can be expressed using whole numbers. However, some academic chemistry and physics books continue to identify the relationship between pressure and temperature as Gay-Lussac’s law.