Table of Contents (click to expand)
Capillary action is the movement of water through a porous material, such as soil, against the force of gravity. The water is drawn up the material by the forces of cohesion and adhesion, which are the attractive forces between the molecules of the water and the molecules of the material.
Water in soil is absorbed by a plant’s roots and propelled upwards to the rest of its organs with absolutely no assistance. The plant, to push along the water the nutrients to replenish its resources, does not issue any force to negate or overcome the pull of gravity. With no force to drive it, how in the world does the water rise against gravity? What sorcery is at play here?

Recommended Video for you:
Capillary Action Definition
The startling rise of a liquid in a narrow tube is called capillary action or simply, capillarity. Capillary action has fascinated people so deeply, in fact, that Einstein’s first paper didn’t explore his esoteric theory of cause and effect or gravity, nor did it demonstrate the particle nature of light. Instead, it described his Conclusions Drawn from Capillary Action, a paper now obscured in the fame of his miracle year papers.
The Irish chemist Robert Boyle, intrigued by the observations of a “few inquisitive French men” as he put it, dipped a thin tube in red wine and witnessed how, unlike mercury, the wine rose to a certain height in the tube. Why do water and wine rise, as the French and Boyle observed, but mercury doesn’t? Eventually, chemists realized that whether a liquid would ascend or descend depends on two forces: the cohesion and adhesion forces between the molecules of the liquid and molecules of the tube.

The Cohesion And Adhesion Forces
The cohesion forces are the attractive forces that, as the name suggests, cause cohesion, or bind the molecules of the liquid together. It is the cohesion forces that power surface tension, the property of a liquid to resist penetration. They exist between similar atoms, such as the branched atoms of hydrogen that constitute water.
Adhesion forces aren’t all that different. These are attractive forces as well, but the attraction they encourage doesn’t manifest between similar atoms, but dissimilar atoms. As their name suggests, they cause adhesion, or glue one family of molecules, in this case, the liquid, to another family of molecules, here, the capillary surface.
A liquid will rise only if the adhesion forces between its molecules and the tube’s molecules are greater than the cohesion forces among its own molecules. A tube, such as a plant’s thin stem carrying water and nutrients, attracts and “pulls” the liquid higher and higher along its adhesive surface.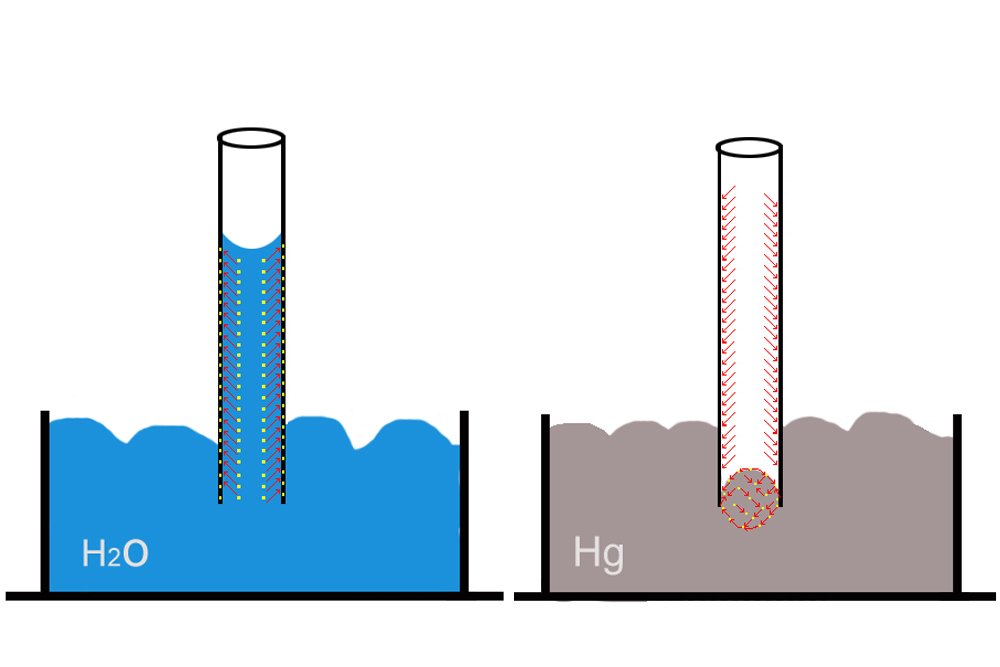
A liquid like mercury does not rise in a glass tube for the simple reason that its cohesion forces are greater than the adhesion forces between it and the glass. In fact, the cohesion forces of mercury are so strong that it exhibits what is called capillary repulsion: it does not rise but, to the contrary, descends lower in the tube! However, it is imperative to understand that capillarity is therefore not only a function of the properties of the liquid but also the chemical composition of the tube.
As the liquid rises, the top of its surface assumes a concave shape. This represents the tussle in which adhesion and cohesion forces are constantly engaged. Eventually, as more and more water enters the tube, the cohesion forces gain sufficient strength to negate the adhesion forces: equilibrium is achieved, which is evident from the flattening of the concave surface.
The Height
The height of capillary rise is determined by this equation:
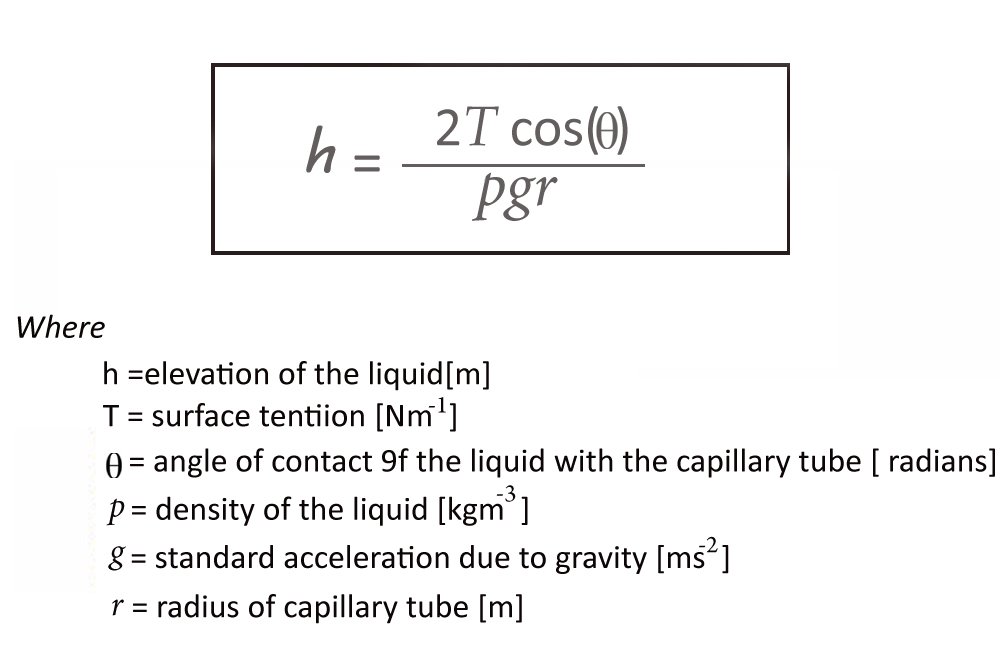
One can discern that the height to which a liquid will rise is inversely proportional to the radius of the tube. The wider the tube, the lesser the ascension. This is because a wider tube will mean a lesser quantity of the liquid is in contact with its surface, which logically causes the adhesion forces to dwindle.
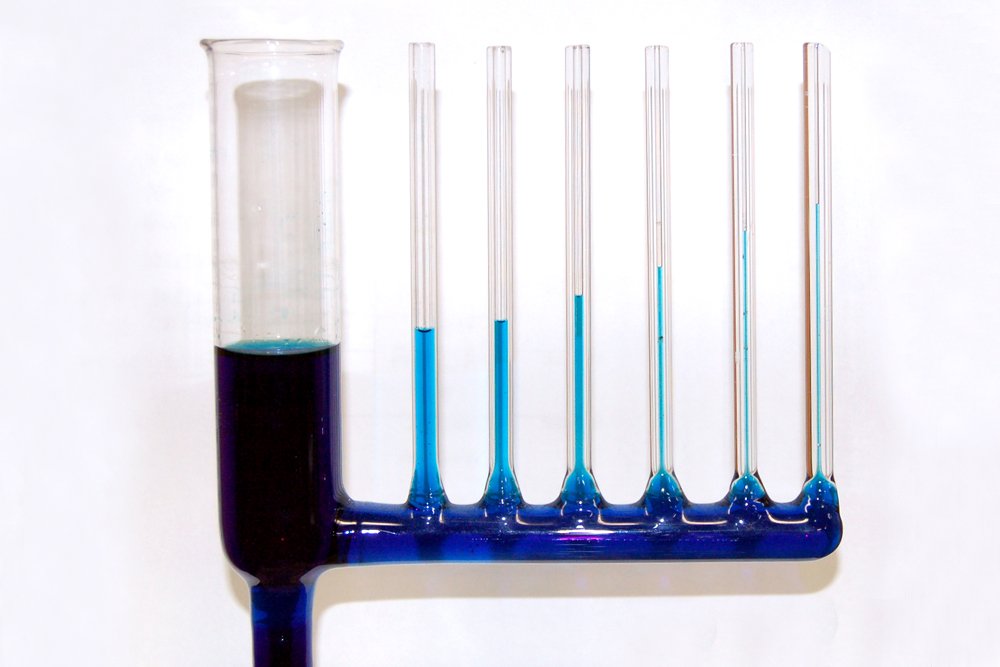
Lastly, while the adhesion forces are initially strong enough to overcome the pull of gravity, gravity will eventually emerge victorious. As the water builds up in the tube, at a certain height, the mass of the liquid becomes too large for the adhesion forces to lift. At this height, the intermolecular forces are utterly defeated by gravitational force.













