Fish osmoregulate through their gills, kidneys and intestines. Fish that live in salty marine waters absorb most of the water they take in and expend energy to excrete the excess salt through their kidneys and gills. Freshwater fish excrete large amounts of water and retain most of the ions, as well as urea.
For those standing on the shore, the sea probably looks like one singular expanse of water, but ask those who call it home and you’ll find that not all water is the same, just like the plains are different from the mountains, even though they’re both made of the “same” earth.
The waters on Earth offer two broad types of homes—freshwater and saltwater. Freshwater has a low salt content, specifically in terms of sodium chloride. Saltwater marine bodies, as the name suggests, is salty. Freshwater bodies have a salt content of less than 1%, while seawater (which is usually salty) has an average salt content of more than 3.5% of the seawater’s weight.
Recommended Video for you:
How Do Fish Deal With The Saltiness Or Lack Of Water, And Why Does Salt Concentration Matter?
First of all, not all fish can handle all levels of salinity. Those fish that cannot handle large changes in salt concentrations are called stenohaline fishes; they prefer the cozy little salt concentrations to which their bodies are physiologically adapted. Fish that can tolerate and adapt to fluctuation in salt levels are called Euryhaline fish.
Your adorable goldfish is a stenohaline fish, preferring its freshwater habitat with very little salt.
On the other hand, salmon and trout are euryhaline fish, living part of their lives in freshwater and then migrating to their marine saltwater habitats.
Despite fish living in water, they can be at risk of becoming dehydrated (or more logically, over-hydrated). To prevent this, fish employ some very interesting tactics.
Dealing With Salt: Osmoregulation
All life is supported by water. As such, all the organic matter we are made of floats or interacts in some way with water. However, there is a critical balance of water and salts that life must maintain. Too much or too little of either and life isn’t happy (or alive). Living things balance their water needs through a process we call osmoregulation–the regulation of osmosis.
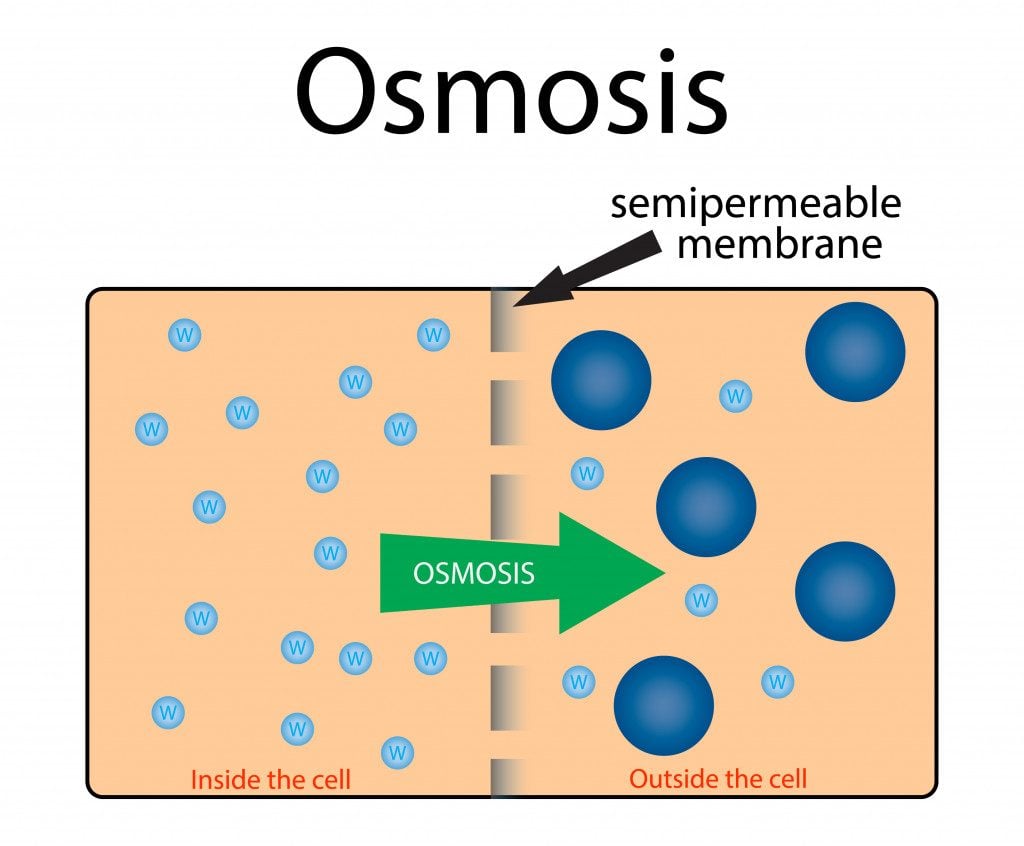
Osmosis is the process of water moving through a semipermeable membrane from a lower concentration solution to a more concentrated solution. To simplify this, osmosis is when water moves from a region with a lot of water to a region that has very little water, passing through a semi-permeable membrane—a membrane that will only allow water or similarly sized molecules to pass through it. Hopefully, this picture will make it easier to understand.
Tonicity
The reason freshwater fish cannot survive in saltwater and vice-versa has a lot to do with a property of any liquid called tonicity. In simple words, it is the ability of a solution to exert osmotic pressure upon a membrane.
Tonicity comes in three types: hypertonic, hypotonic and isotonic. For the scope of this article, we are only interested in the first two.
Hypotonic And Hypertonic Solutions
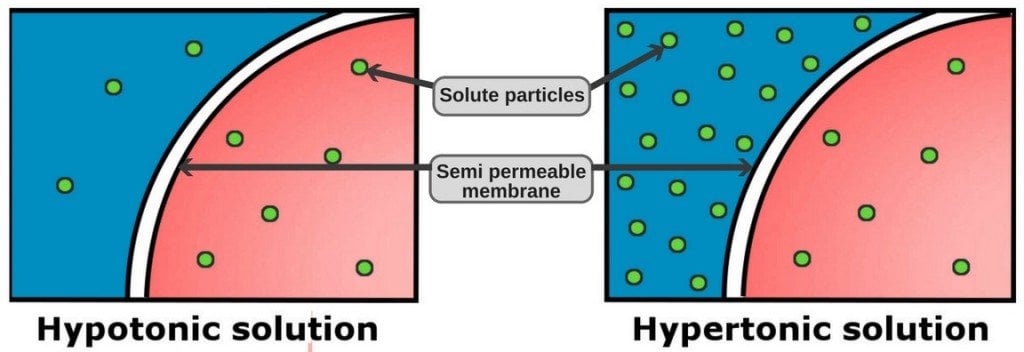
A hypotonic solution has a lower concentration of solutes (solutes are the substances dissolved in a solution, e.g., sugar is the solute in a sugar solution) inside the cell than outside of it. On the other hand, a hypertonic solution has a higher concentration of solutes outside of the cell than inside it.
Back To Osmoregulation: How Freshwater And Saltwater Fish Survive In Their Corresponding Waters
Seawater is hypertonic to the fish living in it, which means that the salt content of the surrounding water is higher than the content inside the fish. As a result, they lose the water inside their body to the surrounding seawater due to osmosis.
On the other hand, freshwater is hypotonic to the fish that live in it, i.e., the salt content in their body is higher than the salt content of the water surrounding them. Due to osmosis, therefore, water continuously flows into their body (the area of high solute concentration, salt being the solute, in this case).

This is where osmoregulation comes in. Fish have a fine-tuned osmoregulation system that prevents marine seawater fish from getting dehydrated through losing a lot of water, and prevents freshwater fish from become over hydrated.
To compensate for this water loss, saltwater fish drink huge amounts of water and are therefore able to survive in highly saline waters. Freshwater fish excrete huge amounts of water to prevent overhydration.
However, simply drinking or excreting this water isn’t enough.
Fish osmoregulate using their kidneys, intestines, and gills (along with a few other structures). All these organs are involved in either excreting or absorbing water and/or ions, so modulating their functioning helps fish adapt to and live happily in their specific surroundings.

The kidneys are obviously very important, and are responsible for excreting the right amount of water and ions to maintain homeostasis. To survive in the face of this continuous supply of water, freshwater fish must urinate very frequently, while marine fish excrete concentrated urea and salts, retaining as much water as they can.
Marine fish and even seabirds, sea turtles and some reptiles have a salt gland that actively removes sodium and chloride from the blood and excretes it as a concentrate solution.
The gills are important osmoregulators. Cells in marine fish called chloride cells or ionocytes, in the gills, produce the ion channels Na+/K+ ATPase that excretes sodium from the gills at the expense of ATP. ATP is required, since the sodium and other ions, are being pumped against the concentration gradient, from a lower concentration of sodium inside the cells to the high sodium concentration of sea water.
Fish, especially euryhaline fishes, can detect changes in salinity in the environment, which triggers a set of physiological and behavioral responses. The osmoregulation in euryhaline fish is fascinating. These fish can adapt to large changes in salinity through some impressive switches in their bodies. Once they sense a change in salinity, they begin to switch between excretion or absorption and their drinking behavior. A variety of proteins are synthesized to deal with the changes and remodel the cellular structure of their gills.
There is a third kind of fish that osmoregulates by matching its internal salt concentrations with those of its surroundings. These fish, called osmoconformers, retain and synthesize urea, rather than excreting it. Since both their extracellular osmolality and the surrounding water’s osmolality is the same, no water is lost from within its cells. Sharks are prime examples of osmoconformers.
Osmoregulation is energetically costly and changing strategies can increase the energy demands of a fish. Ion transport pumps that either excrete or absorb ions from the extracellular fluid or from the environment are dependent on ATP (the energy currency of the body). The process to excrete or retain urea also utilizes energy, especially the latter.
Fish are very sensitive to even the slightest fluctuations in the salinity of the water in which they live. That’s why it’s recommended to fully understand the biological requirements of a fish before putting it into an aquarium at your house!
References (click to expand)
- Cao, Q., Gu, J., Wang, D., Liang, F., Zhang, H., Li, X., & Yin, S. (2018, January 17). Physiological mechanism of osmoregulatory adaptation in anguillid eels. Fish Physiology and Biochemistry. Springer Science and Business Media LLC.
- Salmon Osmoregulation. The University of New Mexico
- OSMOREGULATION, RED DRUM, AND EURYHALINE FISH. University of Kentucky Research and Education Center Botanical Garden
- Kültz, D. (2015, June 1). Physiological mechanisms used by fish to cope with salinity stress. (J. E. Podrabsky, J. H. Stillman, & L. Tomanek, Eds.), Journal of Experimental Biology. The Company of Biologists.
- Cao, Q., Gu, J., Wang, D., Liang, F., Zhang, H., Li, X., & Yin, S. (2018, January 17). Physiological mechanism of osmoregulatory adaptation in anguillid eels. Fish Physiology and Biochemistry. Springer Science and Business Media LLC.