Table of Contents (click to expand)
The Oxford Electric Bell, set up in 1840 and constructed in 1825, has been ringing for nearly 179 years and has rung more than 10 billion times.
When I first heard about a battery that had lasted for over 179 years, my immediate thought was… what if we had a battery like that for our mobile devices? Imagine how many hours we could spend mindlessly scrolling through Instagram or watching cute dog videos on Youtube! The possibilities would be endless…
However, before I fantasized for too long about this game-changing battery, I was notified of a slight problem, namely that neither the scientists nor the owners of the battery know what it’s really made of. What we’re describing is the battery powering the electric bell at the Clarendon Laboratory at Oxford University.

Recommended Video for you:
History Of The Battery
It all started when Luigi Galvani, an Italian physicist, accidentally discovered bioelectricity in the 1780s. Luigi was dissecting a dead frog with some metal tools and noticed something strange. Whenever he struck the frog’s nerves with the metal tools, the frog’s legs flexed. He first attributed this strange behavior to vital fluid, which is linked to an old theory of biology that is no longer accepted in the field. Later on, he changed his mind and said that it must instead be animal electricity.
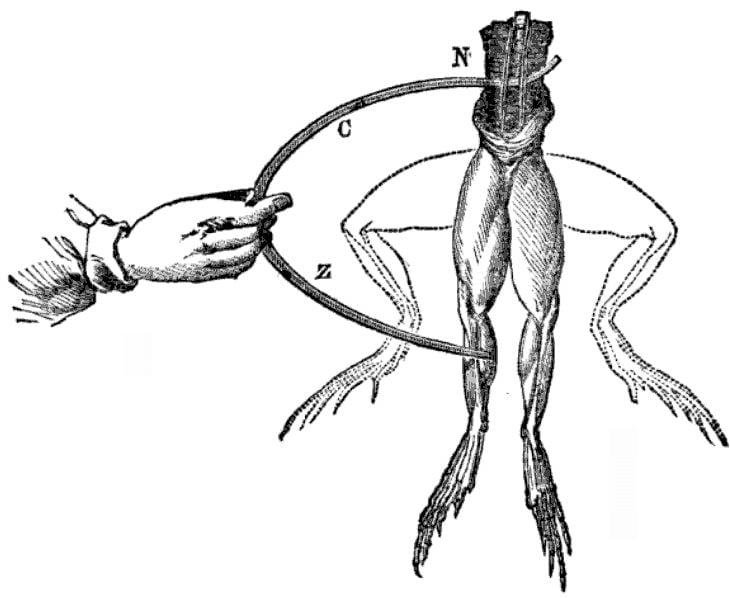
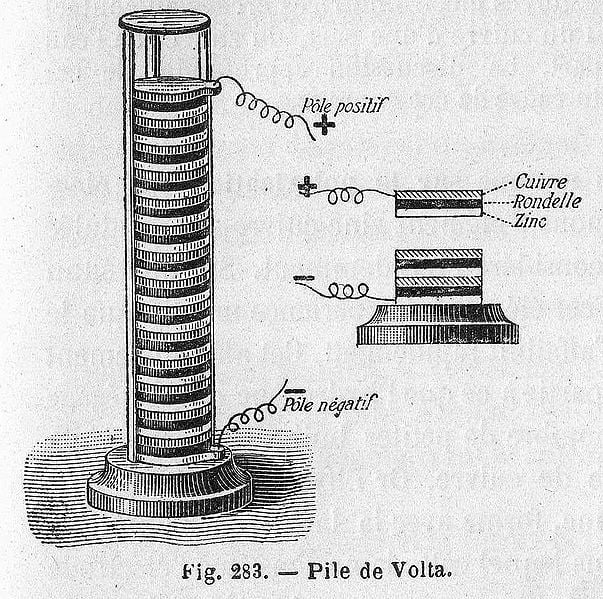
Another Italian physicist, Alessandro Volta, heard of Galvani’s experiment and suggested that both theories were wrong. He believed the legs twitched merely because the nerve acted as an electrolyte, while the electricity was actually generated due to the difference in materials of the tools being used. Volta later proved his theory by creating the first-ever chemical battery in 1800.
The battery was made by piling up discs of copper (or silver) and zinc sandwiched between cardboard discs soaked in brine (or sulfuric acid with water). The battery was called a “Voltaic Pile” or “Dry Pile“. Volta, with the invention of the Voltaic pile, helped to debunk the prevalent theory that electricity is only generated by living beings. However, the battery Volta created had a few disadvantages. It was dangerous to handle due to the sulphuric acid and the power of the cell also diminished after an hour or two.
Giuseppe Zamboni, another Italian physicist, improved the Voltaic Pile and invented his very own battery, the Zamboni Pile, in 1812. The Zamboni Pile utilizes a number of paper discs coated with zinc foil on one side and manganese dioxide on the other. The moisture of the paper discs acts as an electrolyte.
By bringing the terminal ends of the Zamboni Pile close together, and suspending a metal ball between them, an arrangement called an electrostatic clock is formed. The arrangement is called a clock because the metal ball oscillating between the terminals looks like a pendulum. The Oxford Electric Bell has an identical setup to that of an electrostatic clock.
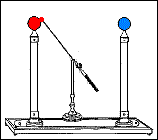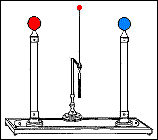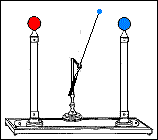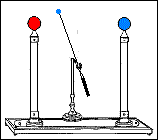
Fast forward to 1825, when London-based instrument makers Watkins and Hill created the Oxford electric bell and the battery to power it. Although created just 25 years after Volta invented the first-ever chemical battery, this battery has outlived every other battery ever made. In fact, this battery currently holds the Guinness World Record for the “Most Durable Battery“.
The Oxford Electric Bell
Created by Watkins and Hill in 1825, the electric bell was brought to Oxford University by Reverend Robert Walker in 1840. The bell currently sits on a shelf in the foyer of the Clarendon Laboratory of Oxford University. The label placed near the bell reads “Set up in 1840” in Robert Walker’s handwriting. Another label reads “Demonstration Dry Pile Purchased 1840”.

The bell setup consists of a spherical metal clapper that oscillates between two small bells. The metal clapper is approximately 4mm in diameter. The metal clapper is powered by the battery placed above the bells. The battery is believed to be a dry pile battery with a paste inside it. This paste contains the minimum amount of water required for the electrolyte to work. A coating of solid sulfur keeps the water inside and helps avoid any leakages. Beyond this, we don’t really know what materials or components are inside the body of the battery.
Now, coming to the most important question… how has such an old battery lasted for so long?
How Does The Oxford Electric Bell Work?
Since we don’t know about the exact interior of the Oxford Electric Bell, there’s only one way for us to answer this question. We must do what scientists always seem to be doing—assuming.
By looking at battery diagrams from around the same time that this one was constructed, back in the 1820s, the battery can be assumed to be a Zamboni pile with around 2000 or more discs composed of zinc and magnesium dioxide. All 2000 discs would be stacked one on top of the other. These 2000 discs create a massive voltage of approximately 2 kilovolts between the two bells. Mind you, that is 16 times greater than America’s main supply voltage. [I’m not sure of America’s main supply voltage. Some websites show it to be 110V, while others set it at 220V], but the metal clapper takes only 1 nano-amp of current each time it rings.
This tiny current requirement of the clapper and massive amount of available voltage is probably why the Oxford Electric Bell is still ringing. In the 179 years since its inception, the bell has rung more than 10 billion times. Yes, you read it right. Billion… with the Big B.

To know exactly what the battery is made of, we would have to cut it open and end the bell’s 179-year-long run, but opening the apparatus would also ruin the experiment and prevent us from seeing how much longer it will last.
Conclusion
The bell has now been placed behind two panes of glass to dampen its continuous ringing. “How long will the Oxford Electric Bell continue ringing?” and “What is the battery inside made of?” are two of the most burning questions in the scientific community. People believe that the bell will eventually stop when the clapper breaks because of wear and tear throughout its prolonged lifetime.
In 1841, just a year after being set up, Watkins and Hill wrote in a letter:
“The residual electrical power sufficient to keep up the ringing of the bells seldom lasts for longer than three or four years. It is a pretty apparatus but also transient in its working process”
They would have been proud to learn just how wrong they were, and I’m sure they are smiling down from the stars with pride to hear their bell still ringing in all its glory.