Table of Contents (click to expand)
Galvanic cells are electrochemical cells that convert chemical energy into electrical energy. They are compact sources of electrical power.
PAT! PAT! PAT! “This remote stops working only when we really want to watch something!” exclaimed Arun’s grandfather as he smashed the TV remote against his hand.
“Stop hitting that, Dad, and put these batteries in first,” said Arun’s mom. Arun saw his mother hand over two objects to his grandpa, and upon loading into the remote, it suddenly worked. Arun was amazed and curious about what those were and why they needed to be replaced.

Isn’t this one of the most common scenarios in a household? Like Arun, most of us as children would have wondered what batteries were. Some of us may have even tried to break them open, just to see what was inside that rigid, hard-to-break shell!
Recommended Video for you:
What Is A Battery?
Batteries are simply a source of electric power. We plug our devices into sockets to get current and get them working. Similarly, batteries are compact sources of electricity. A battery is a package of one or more Galvanic cells that produces a current with the use of chemicals.
Now, what are galvanic cells?
To understand this better, let’s create a small world to learn what they are and how they produce electricity.
Understanding Galvanic Cells Through A Galvanic Empire
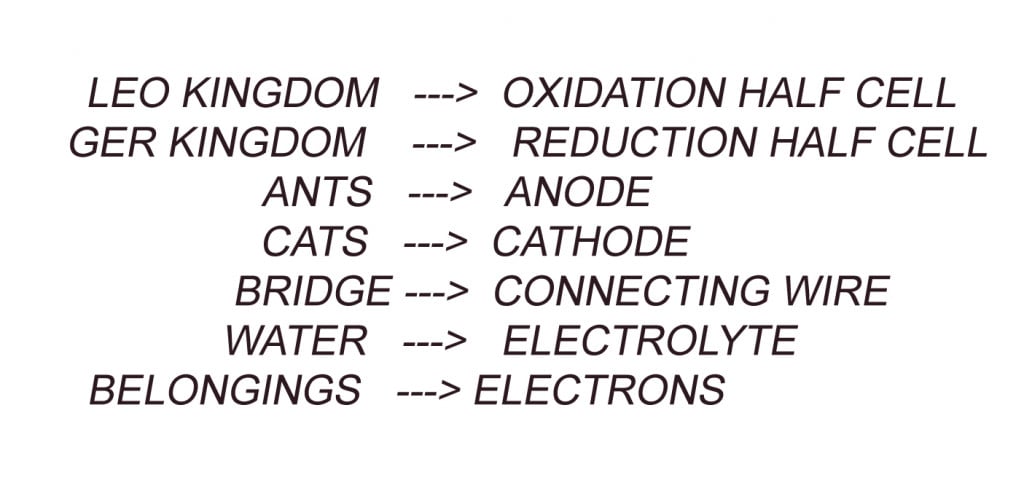
Long, long ago there was an empire called the Galvanic Empire. It consisted of two kingdoms called the LEO kingdom and the GER kingdom. The people of the LEO kingdom were called Ants and the people of the GER kingdom were called Cats.
Both the kingdoms were separated and surrounded by water and huge walls.
However, the two kingdoms could not operate separately. They had to work together to maintain a successful and peaceful empire. Unity is the key! Hence, the kingdoms were connected by a bridge to communicate and transport materials between them.
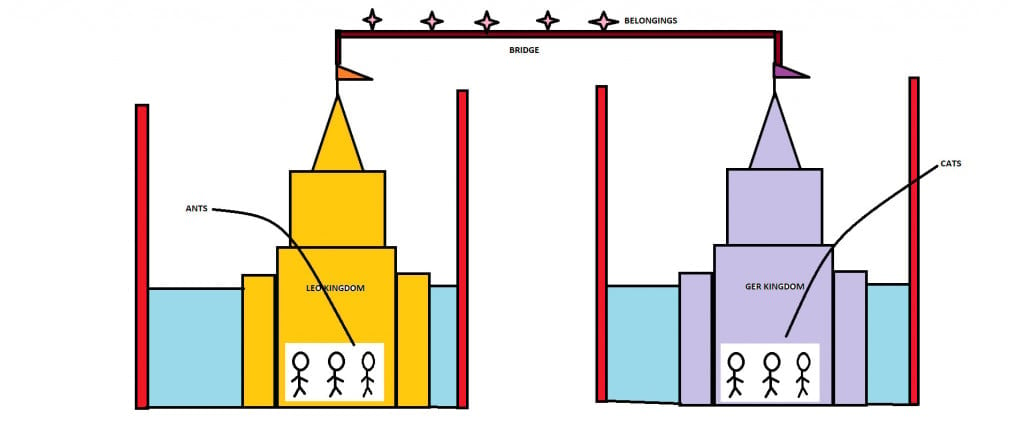
One notable quality of Ants, i.e., the people of LEO, is that they are sacrificial. They lose or sacrifice their “belongings” to the Cats. And these “Belongings” are transported through the connecting bridge. The notable quality among Cats, i.e., the people of the GER kingdom, is that they are acquirers. They accept the “belongings” given by the Ants.
The more the LEO kingdom sacrifices, the more the GER kingdom grows. Sad, but true!
The Ants Shrink while the Cats grow.
As anyone can guess, at some point there will be fewer “belongings” for the Ants to give and for the Cats to gain. Hence, the empires will stop operate.
This is the rise and fall of the Galvanic Empires!
Let’s Put That Into Science!
Now let’s translate that to the real world with the help of this little empire that we created.
A Galvanic cell consists of 2 half cells (referred to as Kingdoms); the oxidation half-cell (LEO kingdom) and the Reduction half-cell (GER Kingdom).
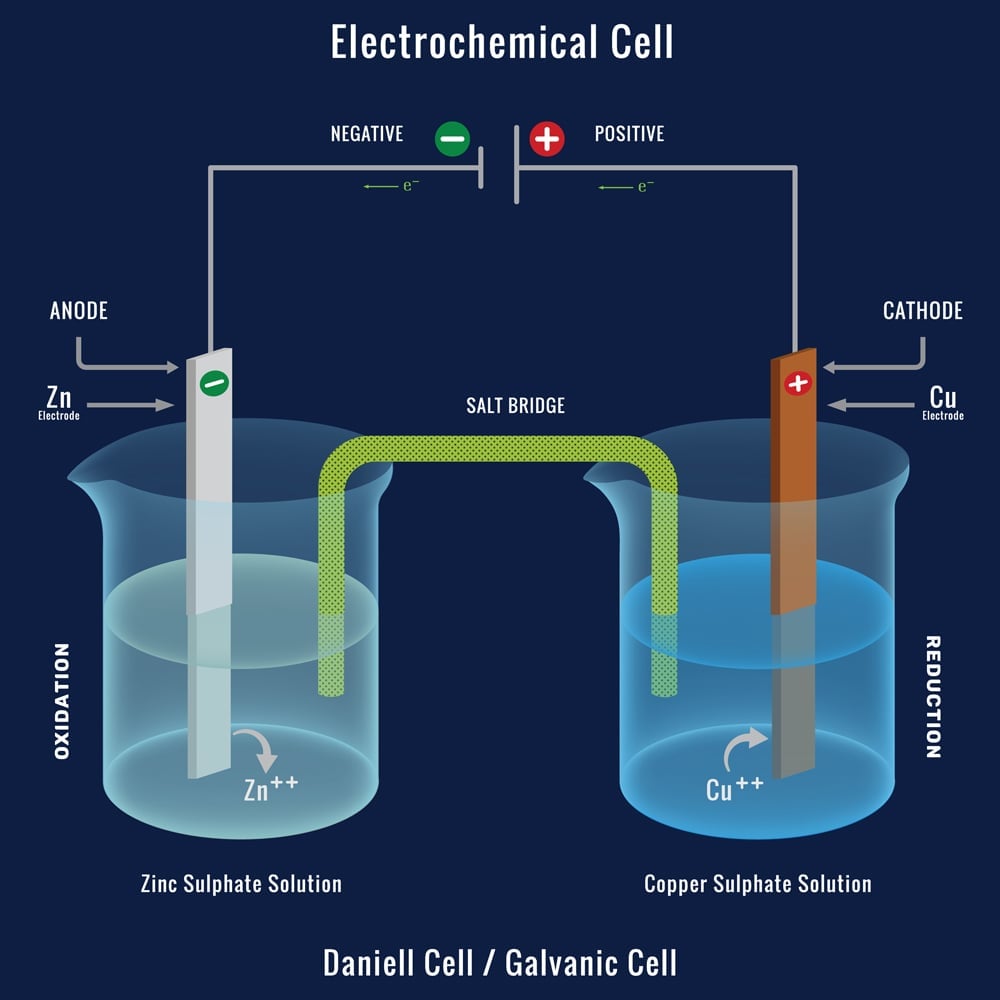
Each half cell consists of a metal (people) dipped into an electrolyte (Water). These two Half cells are connected by a wire (the bridge). In one of the metals, the loss of electrons (sacrifice) takes place, while the other metal gains (Acquires) electrons. These electrons are transported through our connecting wire.
The metal where oxidation (loss of electrons) takes place is called the anode (Ants) and the metal where reduction (Gain of electrons) takes place is called the Cathode (Cats). Oxidation and Reduction cannot take place separately.
Zinc and Copper are commonly used as two electrodes dipped in Zinc sulphate and copper sulphate solutions, respectively. The Zn loses electrons and becomes Zn2+ (Oxidation), whereas the Cu2+ in the copper sulphate solution gains those electrons and becomes Cu.
The Anode corrodes, while the cathode grows. As we are already aware, the movement of these electrons is referred to as current or electricity. This is the simple working of the Galvanic cell.
How do I remember?
It’s a bit confusing to remember, which is where our acronyms come in handy.

Loss of electrons is called oxidation whereas the Gain of electrons is called reduction.
If a current requiring device, such as a bulb, is placed between the connecting wire, it glows due to the flow of electrons. This is how the Galvanic cell works as a source of electric power.
Conclusion
The concept that two metals, when in contact, can produce electricity, was discovered by Luigi Galvani. When two different metals were in contact and when both were touched at the same time to two different parts of a muscle in a frog’s leg, to close the circuit, the frog’s leg contracted.
A year after Galvani published his work (1790), Alessandro Volta showed that the frog was not necessary, using instead a force-based detector and brine-soaked paper (as an electrolyte). Hence, the terms Galvanic cells and Voltaic cells are often interchangeable and refer to the same setup.
Galvanic cells have a wide variety of applications, and are used in watches, clocks, remote controls, phones, cameras, laptops, and so many more common items. Being lightweight, portable, and rechargeable are some of the key advantages of Galvanic cells, which have changed the way we power our lives in countless ways!