Table of Contents (click to expand)
Batteries are typically aligned in opposite directions and next to one another so the current can flow smoothly with a minimal need for additional hardware. When batteries are arranged in a series, the (+) and (-) terminals must be connected; an alternating orientation makes this more efficient and easier to design.
Think back to your childhood, and the joy you felt when opening a brand-new toy—a remote-controlled car, a new stereo, or even a Furby. After tearing off the wrapping paper, the most important things to locate were batteries so you could turn it on and start having fun. However, one thing you undoubtedly noticed, and have seen hundreds of times since, is that the visual instructions for the batteries explicitly told you to align the batteries in opposite directions.

You would meticulously match the nub side of the battery to the (+) sign and the flat side of the battery to the (-) symbol. After clicking the battery panel back into place and flicking a switch, power had been achieved! Placing the batteries in a row in opposite alignments isn’t difficult, of course, but it does beg the question—why are batteries designed like that?
Recommended Video for you:
What Is A Battery?
Before we can understand the nuances of battery and product design integration, we should take a quick review of batteries themselves. A battery, quite simply, is a collection of electrochemical cells with external connections that enable it to power electronic devices. From minute batteries for hearing aids and smartphones to the massive batteries under the hood of your car, there are some basic elements of a battery that remain the same.
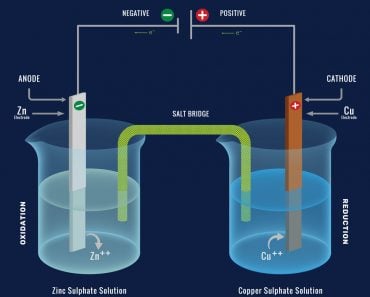
While a battery is in use, the positive terminal is the cathode, and the negative terminal is the anode. Electrons flow from an external circuit into the anode and move to the cathode (the positive terminal). A redox reaction occurs that will convert high-energy reactants into low-energy products; the remaining energy is then available for use by the device or product in the form of electric energy, which powers the device.

Basically, chemical energy within the battery is converted into electrical energy in the form of direct current (DC). The chemicals contained within the battery are typically acids, and are referred to as the electrolyte. Batteries themselves may be made of nickel, lithium, cadmium, lead, mercury and alkaline, all of which have different pros and cons. Today, lithium ion batteries have become incredibly popular due to their high energy density, allowing them to provide a lot of power from a small source, and being highly rechargeable.

In the past, a battery referred to multiple cells connected to one another, but a modern battery can now contain several cells within itself. However, when multiple batteries are used together, they are typically arranged in series, allowing them to double both their voltage and Amp-hour (the amount of energy charge in a battery that will allow one ampere of current to flow for one hour). The other option for batteries is to be arranged in parallel (increasing Amp-hour, but keeping voltage the same), which isn’t appropriate for devices that may require a higher voltage amount. To learn more about the nuances of series and parallel circuits, check out our in-depth article here.
Efficient Battery Design
When multiple batteries are arranged in series, the positive and negative terminals of adjacent terminals must be connected; that is how current is able to flow, completing the circuit and powering the device. With that in mind, arranging batteries in alternating pattern—a batter with the (+) terminal facing up, followed by a battery with the (-) terminal facing up—is efficient.
If the batteries were arranged with like terminals all facing the same direction, a wire would need to be included in the design between the positive and negative terminals on neighboring batteries. However, with the batteries alternating in terms of their terminal direction, a small metal plate at either end of the battery can establish this essential connection (between the (+) and (-) terminals).

Depending on the product design, the batteries may have to be arranged next to one another, or in a row. In the latter arrangement, often found in flashlights, the positive and negative terminals are compressed and held against one another, allowing for the current to smoothly flow. In other products, such as handheld video games, tightly packing multiple batteries beside one another in alternating directions is the most efficient design in terms of circuit completion and total volume.
A Final Word
When designing a product, every millimeter needs to be considered; even the inclusion of one extra wire is seen as wasteful, and may add to the final cost of production. Manufacturers and designers are perpetually seeking cost-efficiency, without compromising functionality. Arranging batteries in this up-down pattern, and linking the positive and negative terminals with a metal bar, rather than a wire, improves the efficiency and performance of the design. In terms of the battery orientation themselves (in a row or adjacent), that is dependent on the overall shape and other physical components of the product.
References (click to expand)
- Scrosati, B. (2011, May 4). History of lithium batteries. Journal of Solid State Electrochemistry. Springer Science and Business Media LLC.
- About Batteries - MST.edu. Missouri University of Science and Technology
- Batteries: Electricity though chemical reactions. LibreTexts
- Batteries - Hyperphysics. Georgia State University