Soil acidity and alkalinity are a function of the amount of acid and base cations present in the soil.
While planning to sow a new plant, your local gardener would advise you to plant according to the fertility of the soil in your backyard. This may appear to be a trivial detail, but the nature of the soil is the major factor responsible for the quality of produce growing from it. Farmers and agriculturists are always on the lookout for ways to control the quality of soil. After all, it is the source of our food!
Soil is either acidic, alkaline or neutral in the natural world. The nature of the soil is checked by testing its pH value. The pH scale measures hydrogen ion concentration in the soil. To understand how a pH scale works, you can read this article explaining how to measure soil pH.
Recommended Video for you:
What Are Soil Acidity And Alkalinity?
Soil is said to be acidic when there is an increase in the concentration of acidic cations, such as aluminum (Al3+) and hydrogen (H+). Such soil falls below the range of 6.5 on the pH scale. On the contrary, alkaline soil ranges above 7 on the scale. It is primarily composed of base cations like sodium.
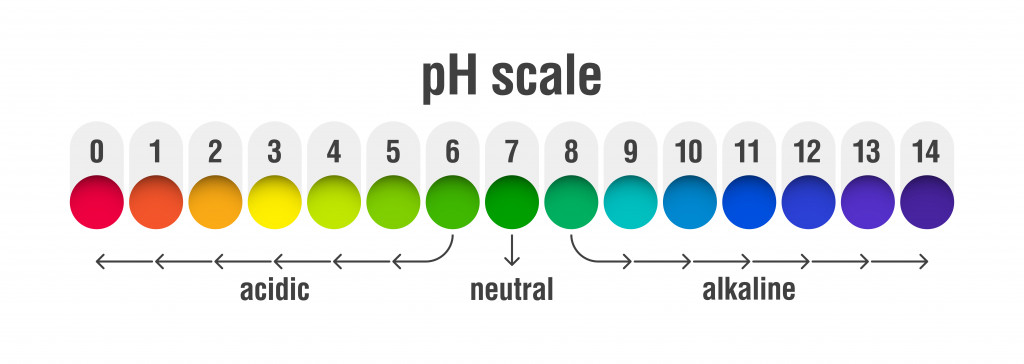
Cations are species that carry a positive charge and can accept a pair of electrons. The terms “base” and “acid” describe the impact of the specific cation on soil pH. Soil with a high amount of acid cations stored in it will have a low pH. In contrast, base cations make up the majority of alkaline soil.
What Are The Factors Affecting Soil Acidity?
There are various things that affect soil acidity. Soil can turn acidic due to variations in the chemical makeup of the parent materials. For example, soil formed from granite is probably more acidic than soil formed from limestone. Heavy rainfall can also eliminate basic cations from the soil.
That’s why sandy soils usually experience acidification, as water percolates through them quickly.
Another reason for an increase in H+ ions in soil is decaying organic matter. Water in the soil combines with the carbon dioxide (CO2) released from decomposing organic matter to produce carbonic acid. However, like the nature of parent rock and heavy rainfall, decomposing organic matter also alters soil acidity over long periods.
Farming practices and the use of ammonium-based fertilizers are crucial concerns. Crop harvesting affects soil acidity because crops need to take in cations for nourishment. To maintain electrical equilibrium, a hydrogen ion with a positive charge is released into the soil. Harvesting plants removes that crop from the soil and causes a deficit of alkalinity in the cycle. The remaining hydrogen ions in the soil turn it more acidic over time as this process continues.

Ammonium nitrogen from fertilizer is easily transformed to nitrate and H+ ions through the action of microbes. Depending on the fertilizer, the concentration of hydrogen ions added to the soil differs. To preserve electrical equilibrium, plants emit a negatively charged hydroxide ion when they absorb negatively charged nitrate. In the soil, this hydroxide ion reacts with H+ ions to create water. The hydrogen ion is no longer available to add to soil acidity. If the nitrate ion leaches away from the soil, hydrogen ions are left behind, which lowers the soil pH.
What Turns Soil Alkaline?
Gardening enthusiasts in Utah can only dream of sowing plants like blueberries, which thrive in acidic environments. This is because of the high calcite (calcium carbonate) content in the soils. It is more challenging for plants to absorb essential nutrients from alkaline soil than from acidic soil, due to its lower solubility.
The type of parent material (rock) and the weathering mechanisms involved both affect the pH of natural soil. Rocks rich in calcium carbonate weather and degrade over time, resulting in the formation of alkaline soil. Depending on the location, the soil is often treated with hard water, which contains a high mineral concentration of magnesium and calcium carbonate. This can be reflected in a high pH. Sweet soil is also the result of low rainfall conditions. The salt concentration of soil rises in arid or desert environments where water evaporates rapidly, gradually making the soil more alkaline.
How Does Soil pH Affect Plant Growth?
Soil pH determines the amount of nutrients that dissolve in the soil and are therefore available to plants. However, the ideal condition for plant growth is when soil pH is close to neutral.

The main reason why crops fail in acidic soils is because of toxic substances like aluminum and manganese. These elements remain in a solid state at a pH above 5.5, but start to dissolve in the soil as the acidity increases. The concentration of aluminum cations (Al3+) in the soil can increase 1000-fold with a drop from pH 5.5 to 4.5. Due to this, certain crops may appear to be doing exceptionally well, but will fail altogether with only a slight shift in pH. For instance, wheat may thrive at a pH of 5.0, but it typically fails at 4.0 pH. This is known as element toxicity.
Nutrient deficits including those of zinc, copper, boron and manganese, are an issue in alkaline soils. Sodium concentrations are expected to be high in soils with pH values that are quite alkaline (higher than 9).
How Do Farmers Correct Soil pH?
For acidic soils, the easiest (and most cost-effective) way to reduce the negative effects of poisonous substances is to “lime” the soil. Liming is the treatment of soil with calcium- and magnesium-rich minerals like dolomite and lime. It increases soil pH and causes toxic elements to return to their solid chemical forms from the soil solution.

To deal with issues of high pH, fertilizers that increase soil acidity are the solution. These substances include powdered sulfur and some ammonium-based nitrogen fertilizers.
Different locations will have differences in the nature of the soil due to the various geological and human factors acting on the soil. It is important to study and understand the nutrients available in the soil before deciding on the crops that should be grown there.
References (click to expand)
- Cause and Effects of Soil Acidity | Oklahoma State University. Oklahoma State University–Stillwater
- Why are my soils so alkaline? Can I lower my soil's pH? | USU. Utah State University
- Soil-Nutrient Relationships - CTAHR. The University of Hawaiʻi System
- Soil acidification | Environment, land and water. Queensland
- Causes of soil acidity - Department of Agriculture and Food. agric.wa.gov.au