Table of Contents (click to expand)
Shaving creams contain surfactants or surface-agents which produce several bubbles that are durable and less soluble in water creating long-lasting lather.
Shaving wasn’t always as easy or pleasant (read: bloodless) as it is today. Back in the day, you couldn’t simply pick up a razor and slide it across your face without frequently scraping off chunks of your skin. In fact, you couldn’t just wake up in the morning and decide to give yourself a shave. People didn’t have the lubricating shave gel or safety razors of today that ever so gently dance along the contours of your face.
You had one straight razor (super sharp) that you had to use with the same focus you would need to thread a needle. One wrong move, one incorrect tilt of the wrist, one jerk of the hand, and SLICE! Blood everywhere.
Back then, you would pay a professional to do it for you.

Recommended Video for you:
Surfactants
However, the process and chemical composition have come a long way from how things used to be. Nowadays, shaving formulations come packed with special molecules known as surfactants, which make up the secret sauce allowing for dense, fluffy and longer-lasting lather.
The Origin Of Shaving Cream
Hair has about the same tensile strength as copper wire, but it can be softened by soaking.
Therefore, initially, consumers used water and regular body soaps to soften their hair and lubricate the skin before a shave. Problem solved, right? Wrong! When you try to shave using regular soap and water, you just can’t get the same level of comfort. The water evaporates too quickly, while the soap creates a lather that dissolves in water almost instantly. What you’re left with are semi-wet cheeks and an acute case of razor burn. Inventors from around the world got busy trying to fix this pressing and widespread issue. Consequently, they came up with shaving foams, creams, and gels, which stayed on the skin longer, softened the hair and made shaving an experience that people would look forward to, rather than an ordeal to tolerate.

The first known shaving patent was filed in 1937 by inventors Kroper Hugo and Thomae Erich. Surprisingly, this wasn’t a patent for making shaves more comfortable, but rather ensuring that they weren’t lethal. Hugo and Erich had devised a formula that used certain extracts from soybean that could be mixed with the ‘shaving soaps’ used back then. These extracts were known to have hemostatic properties, i.e., they could stop you from bleeding out in the case of a cut. Thankfully, shaving products have improved exponentially since then. Now there are shaving creams that lather up, fit into pressurized cans, come out as a hot foam, feature anti-bacterial properties, or even have built-in skin softeners and perfumes. However, let’s not get ahead of ourselves about the miracle of shaving. Let’s try to understand what chemical sorcery actually enabled such innovation within our daily shave routine.
How Do Shaving Creams Work?
Essentially, shaving formulations are made of complex molecules known as surface-agents, or ‘Surfactants’. These are molecules that possess a special property of being able to hold on to both oil and water. They’re composed of two parts: a hydrophilic (water-loving) head and a hydrophobic (water-hating/oil-loving) tail.
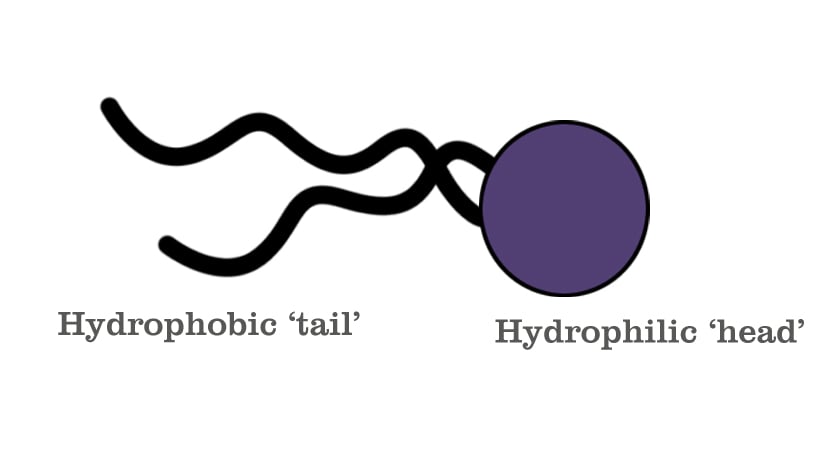
The hydrophilic ion is the one that generates lather. In earlier versions of shaving formulations (in the 1970s), this was usually a sodium (Na+) or potassium (K+) ion. However, both of these are readily soluble in water and therefore get washed away easily. More recently, triethanolamine, which is a much larger ion, was found to be a more durable alternative. This substance reacts with water to produce a large number of tiny bubbles that create the thick, Santa Claus beard-like lather. Meanwhile, the long-chain fatty acid, the tail—usually stearic acid—is responsible for making sure the molecule is less soluble in water, thus allowing the lather to last longer.
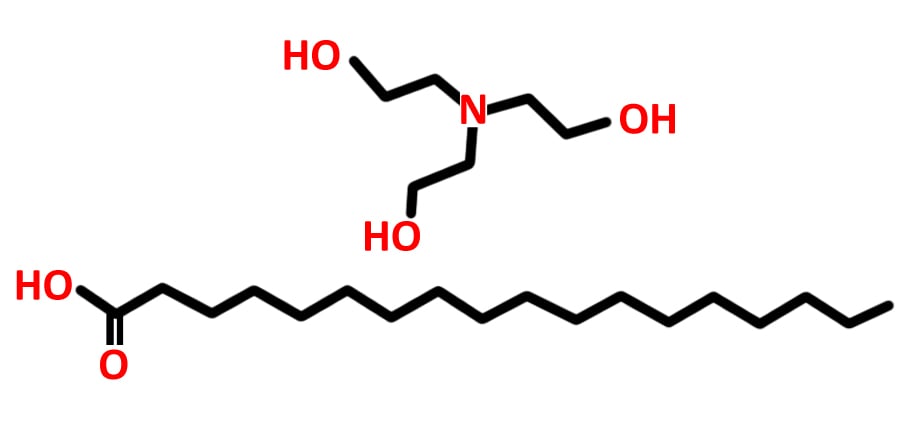
The longer the tail, the longer the lather lasts. Longer chains ensure that the hydrophobic part of the surfactant has greater influence over the entire molecule, thus lending it greater resistance to dissolution in water. The longer acid allows for the shaving formulation to have thicker, more structurally sound bubbles that will stay on your face, even if you like to take your sweet time after a shower. Some earlier versions of shaving cream also used lauric acid, which had a chain of 12 carbon atoms, as it was easier to synthesize. However, all shaving formulations eventually moved to use steric acid, which possesses a chain of 18 carbon atoms.
How Do Shaving Creams Turn To Foam?
For shaving creams to foam up and get that extra fluffy texture, most basic shaving formulations are packed under pressure with a propellant like iso-butane. When you press the button on top of the bottle, the pressure is released, causing the propellant to expand and the formulation to rush up the bottle, where it comes out as a foam. In fact, the iso-butane is also responsible for lending some extra bubbles to the mix, which is the cause behind the excessive fluffiness. Moreover, in 2006, DEB Inc. invented a silicon-based surfactant formula that foams up just by coming in contact with air!

Antibacterial Shave
Some shaving formulations also include glycerol or magnesium aluminum silicates, which provide even greater lubrication for a smoother shave. Inventor Navin Geria from Eveready Battery Co. went a step further and patented an antibacterial shave formulation. This contained the usual surfactants, along with dichlorobenzene alcohol, which provided antibacterial protection. In 1999, Essien Eyo-Okon Ita updated this idea when they recommended using salicylic acid combined with glycerine and alcohol to reduce post-shave skin irritation.
Basically, a lot of research, blood, sweat and tears have gone into making your seemingly mundane morning shave routine as comfortable as it is today. From splashing water on your face and hoping for the best to using sophisticated formulations with enhanced lubrication and antibacterial properties, shaving has come a very long way. Hopefully you think of that the next time you dance around in front of the mirror decked up like Santa Claus before a big night out!












