Table of Contents (click to expand)
- What Is Chloroform?
- Where Is Chloroform Normally Found?
- What Does Chloroform Smell Like?
- Use Of Chloroform: What Is Chloroform Used For?
- Chloroform Effects: What Does Chloroform Do?
- How Does Chloroform Work On The Human Body?
- How Long Does It Take For Chloroform To Work?
- How Long Does Chloroform Keep A Person Unconscious?
- Chloroform Risks: Hazards Associated With The Ingestion/consumption Of Chloroform
- Can You Be Killed By Chloroform?
- Is It Legal To Have Chloroform?
- What To Do If You Are Exposed To Chloroform?
Chloroform is a colorless, sweet-smelling liquid with the IUPAC name trichloromethane and the chemical formula CHCl3. Chloroform is used as a solvent in the paper, construction and wood processing industries as well as in pesticide production. Chloroform can also numb or render people unconscious in small doses.
Pick a famous crime story, serial killer investigation or espionage movie and chances are that the following scene will unfold one way or another:
A villain sneaks behind a target and puts a rag over his mouth; after a few moments, the victim becomes weak on his knees and loses consciousness.
If you’re more interested in chemistry than action scenes, then this question has surely come into your head at some point: Does chloroform really knock someone out that quickly?
Recommended Video for you:
What Is Chloroform?
Chloroform is a colorless, sweet-smelling organic compound with the IUPAC name trichloromethane and the chemical formula CHCl3. It is a dense liquid with tetrahedral molecular geometry with C3 symmetry. The structural formula of chloroform is given below:
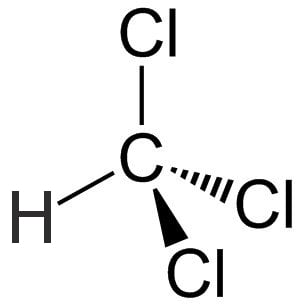
Chloroform is a highly volatile liquid that has been widely used throughout history due to its narcotic properties and has a reputation for anaesthetizing or rendering people unconscious, even when consumed in small doses.
In chemical jargon, chloroform is liquid trichloromethane and is produced on an industrial scale by heating a mixture of chlorine and either chloromethane or methane.
Where Is Chloroform Normally Found?
Chloroform is a naturally occurring organic compound found in the air and in coastal waters, lakes, inland waters and groundwater. However, most of the chloroform found in the environment is produced by humans. Higher chloroform levels are found in industrial areas and in the air above swimming pools where the water has been disinfected with chlorine.
What Does Chloroform Smell Like?
Chloroform is a sweet-smelling liquid, similar to ether, along with a slightly sweet taste. Some people compare the smell to the smell of disinfectants, similar to the smell that is perceived in hospitals and medical facilities. We interviewed a number of chemists working in chemical laboratories who explained that the chloroform smell vaguely resembles the smell of acetone, an organic compound.
Use Of Chloroform: What Is Chloroform Used For?
- Chloroform is often used as a solvent in the chemical production of compounds.
- It is used in the paper, construction and woodworking industries.
- It is used in pesticide and film production.
- Chloroform is used to produce a refrigerant called Fluorocarbon 22.
- Chloroform is used as a solvent in floor polishes, lacquers, adhesives, resins, oils, alkaloids, fats and rubber.
Use Of Chloroform As An Anesthetic In The Past
Chloroform was first used as an anesthetic in 1847 by an obstetrician named James Young Simpson; he actually used it as an anesthetic on two people. A few days later, it was successfully used in a dental procedure in Edinburgh with no discernible adverse effects.
Soon, its popularity as an anesthetic soared to the point that it said that it was even used during the birth of Queen Victoria’s last two children in the 1850s. Its golden age, however, was short-lived, as it was gradually replaced by ether that was much safer than chloroform, and had virtually no side effects.
Chloroform Effects: What Does Chloroform Do?
The effect of chloroform on humans increases proportionally with its dosage. In small doses, chloroform can cause you to feel lethargic and disoriented, but with increasing dosage, you can quickly lose consciousness and feel no pain or sensation. In higher doses, it can lead to tense breathing, complete muscle relaxation and paralysis of the chest muscles, which can often be fatal.
The effects of chloroform on the human body largely depend on its dosage and method of administration.
According to the Wisconsin Department of Health, “immediately or shortly after exposure to a chloroform level of 100 ppm (100,000 ppbv) in the air, a person may feel tired, dizzy, and have a headache.”
Chloroform is known for its anesthetic properties. If taken in small doses, it can numb or knock a person over, while in high concentrations it can be fatal.
There is some evidence that chloroform directly affects the central nervous system, along with the liver and kidneys; at high doses, it can cause respiratory depression and coma.
Although many of us associate chloroform with “a fluid in a rag that knocks people out,” its effects on the human body can be far more complex, and if not carefully monitored, chloroform can be fatal.
How Does Chloroform Work On The Human Body?
When inhaled, chloroform in the lungs passes quickly into the bloodstream. It then travels to the brain and tissues, where it slows and sedates the central nervous system (CNS). It effectively stops communication between the brain and body, causing unconsciousness.
The exact mechanism of how chloroform interacts with the CNS to produce this effect is not wholly understood. However, experts suggest that chloroform, and other anesthetics alike, interfere with the normal firing of neurons in the brain by disrupting the function of fatty molecules that make up the neuron cell membranes. Since messages are sent along neurons as electrical impulses, this disruption prevents normal communication between different parts of the brain, leading to unconsciousness.
How Long Does It Take For Chloroform To Work?
In 1865, The Lancet, the medical journal, called upon any person, criminal or not, to prove that waving a chloroform drenched handkerchief was enough to knock someone out. No one has to date come forward with an answer.
While the right dose of chloroform soaked in a rag can definitely render you unconscious (the Lancet articles cites 5 minutes and persistance should knock someone out, but no experimental evidence was provided), it would take much longer than what they show in movies: you wouldn’t drop unconscious just by taking a whiff!
Issues Of Volatility
Chloroform is a volatile liquid, so it quickly loses its effectiveness when it comes into contact with air. Therefore, it is not a plausible scenario that the “villain holds a cloth soaked in chloroform while waiting for the victim to appear,” since the chloroform in the cloth would lose its effectiveness by the time it is actually pressed against the victim’s nose.
It is possible that a victim in such a situation will not faint simply because of the chloroform. Along with chloroform, the victim may faint due to suffocation, since putting a cloth over the nose and mouth would not allow the victim to breathe.
How Long Does Chloroform Keep A Person Unconscious?
Chloroform, if inhaled in very small doses, can keep a person unconscious from 20 minutes to 2 hours or even more, depending on how concentrated the dose is. Even when the person recovers, they may have symptoms like disorientation, vomiting, headache etc. If the dose is too concentrated, the person inhaling chloroform may potentially die. This is why chloroform is considered too dangerous to be used as an anesthetic.
Chloroform Risks: Hazards Associated With The Ingestion/consumption Of Chloroform
When ingested, chloroform is converted into a chemical called phosgene. Phosgene is toxic to cells, so the use of too much chloroform could cause cells to die.
If you look at how its use is portrayed in films and television, you might assume that it is just another liquid that is harmless to the victim, but that is absolutely wrong.
Some studies have shown a possible link between chloroform in chlorinated water and the occurrence of cancer of the colon and urinary bladder. Liver and kidney cancer developed in rats and mice who ate food or drank water containing large amounts of chloroform for long periods of time.
Can You Be Killed By Chloroform?
Chloroform can be very dangerous, to the point of being fatal to the victim if an inappropriate dose is administered or if the chloroform-soaked cloth is placed too tightly on their face.
For good reason, chloroform is no longer used as an anesthetic; it is a difficult task to determine the right dose that would render a person unconscious without affecting other vital nerve functions.
Is It Legal To Have Chloroform?
In many countries, the government regulates the use and possession of Chloroform.
In the United States, possessing or using chloroform without a valid license is illegal. It is necessary to check with local laws before acquiring the substance. Chloroform is a controlled substance classified as a Schedule II drug by the government in US.
What To Do If You Are Exposed To Chloroform?
The first thing to do is to move away from the source of exposure as quickly as possible.
If the person exposed is already unconscious and unable to move independently, they should be removed from the source of chloroform exposure by others. Clothes that may have come into contact with chloroform should be removed and thrown away. Eyes and skin exposed to chloroform should be washed and rinsed with clean, uncontaminated water.
References (click to expand)
- Anesthesia and Queen Victoria - www.ph.ucla.edu
- Chloroform | Wisconsin Department of Health Services. The Wisconsin Department of Health Services
- (1865, October). Medical Annotations. The Lancet. Elsevier BV.
- Payne, J. P. (1998, July). The criminal use of chloroform. Anaesthesia. Wiley.
- Chloroform | NIOSH | CDC. Ease Control And Prevention
- How does anesthesia work?.