Bones are some of the most resilient parts of the body and burn at significantly higher temperatures than the rest of the body. Normal fires don’t reach temperatures high enough to burn bone.
Shows such as CSI, Dexter, Partners for Justice, and Detective Conan are classics for any avid lover of the mystery and crime genres. Unraveling the case alongside the detectives is what makes the experience of watching these shows so exciting.
However, what lies at the crux of solving many of these highly efficient and seemingly impossible crimes is forensics. Be it a car crash, an apartment on fire, or an unearthed body, forensic experts will give one all the necessary clues to catch the killer.
This leads to the natural question, how is it that even in a car crash or a burnt apartment, they’re able to retrieve bones for the investigation? Why do bones take so long to decompose? What makes them burn-resistant?

Recommended Video for you:
Biology Of Bones
There are a total of 206 bones in the adult human body. There are long bones, short bones, flat bones, and irregular bones within us. They fuse and change over time to produce the skeleton we see and rely on every day.
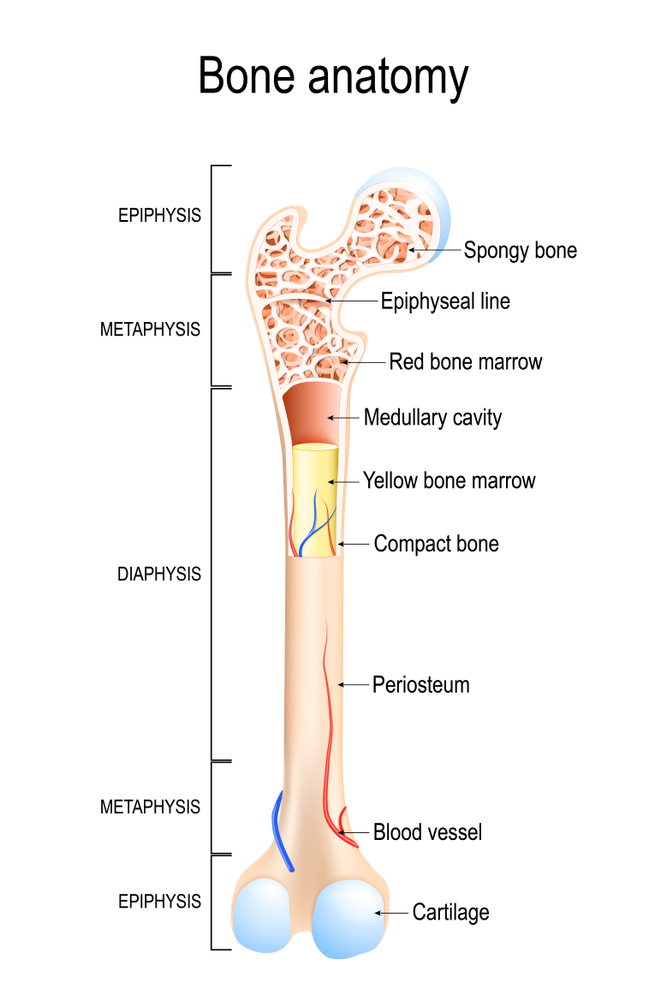
The composition of these bones is complex. Bone is largely formed of minerals, primarily calcium, found as the chemical hydroxyapatite. Associated with the minerals are the organic components: collagen, non-collagenous proteins, lipids (fats), and water.
How much of each substance exists in the body is dependent on various factors, such as age, site of the bone, ethnicity, a person’s physical health, and gender.
What Happens When The Body Comes In Contact With Heat?
The skeleton is covered by skin and tissue intersected by nerves and blood vessels. Using the burn guide provided by the National Institute of Health, we can classify damage by heat into 6 degrees. First- and second-degree burns damage the upper layers of the skin—’the epidermis’. Something like getting a sunburn, which is easily healed, can be included in this category.
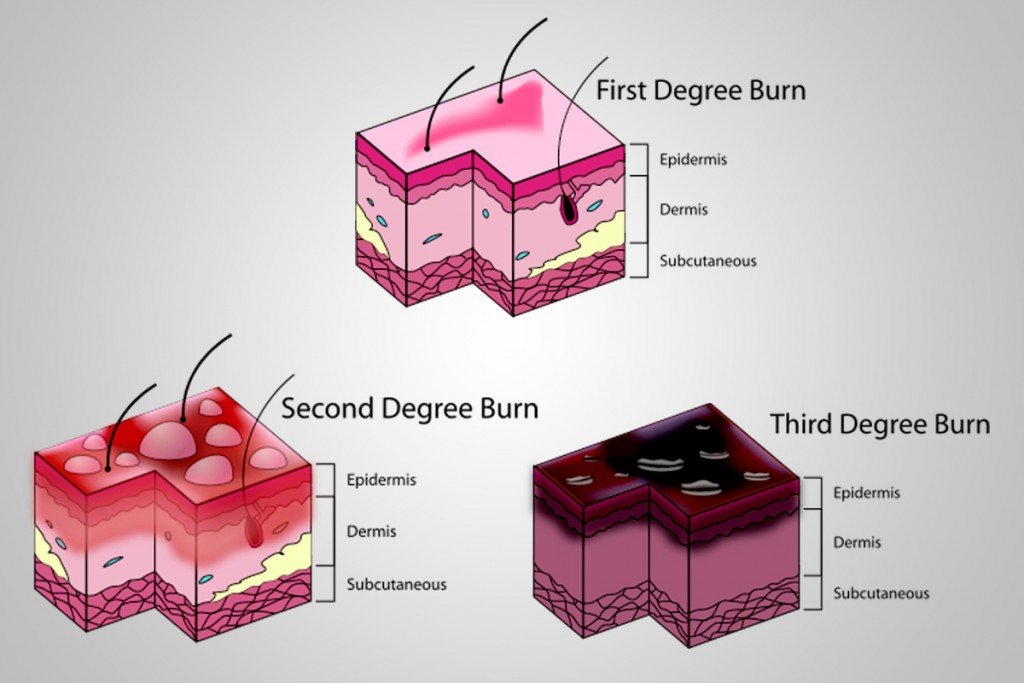
Third-degree burns damage the deeper layers, destroying sweat glands and hair follicles. Usually, these are caused by scalding hot water or oil. Exposure to temperatures ranging from 48°C to 200°C for as little as 5 minutes will produce this result.
Fourth- and fifth-degree burns will damage the remaining fat and muscle, respectively, exposing the bone to damage.
Finally, a sixth-degree burn would damage the bone. The bone consists of two types of collagen—non-calcified and calcified. In simple words, it refers to whether the bone has calcium as part of its structure or not.
Studies have noted that at lower temperatures of 43°C, non-calcified collagen begins breaking down. However, calcified collagen takes around 1 hour of heating at 150°C to denature irreversibly.
Once the organic matter is burnt away, the only component left is the mineral part: hydroxyapatite.
What Is Hydroxyapatite?
Hydroxyapatite or HA is a naturally occurring form of calcium. Bone and teeth are composed of this material. It is used as a replacement for Plaster of Paris, a.k.a POP, as a safer type of bioceramic.
Hydroxyapatite has the chemical formula (Ca10(PO4)6(OH)2). It can be broken into three component compounds–Water, Phosphoric acid, and Calcium.
What Happens When Bones Are Set On Fire?

Hydroxyapatite is a resilient compound and begins to break down at temperatures above 800°C.
In 1998, a study heated hydroxyapatite (commercially produced) to see how its chemical composition changed under heat. They began to see changes in its chemical composition starting at 1000°C. This is roughly the same temperature as the Kīlauea lava recorded in 2016.
At a 1000°C, the chemical loses its moisture and breaks down into calcium phosphates. At approximately 1500°C, the bone has broken down into different components: water, calcium oxide and a compound called alpha-tricalcium phosphate (α-TCP) (as found by a study published in 1999).
A study published in 2013 noted that hydroxyapatite could break down when heated in two ways. The first route involves the formation of a chemical TTCP (tetracalcium phosphate) and then β-TCP (beta-tricalcium phosphate). This eventually converts into α-TCP around 1100°C. In the second route, the hydroxyapatite is directly converted to calcium oxide and α-TCP.
Many of these studies were performed using synthetic hydroxyapatite. However, within a body, the bones interact with many other compounds, which could mean that the temperature at which the bones break down might differ.
Crematoriums use incinerators that reach temperatures ranging from 760-982°C. As mentioned in previous texts, temperatures of 1100°C and above are required for complete bone breakdown. Thus, incinerators only destroy organic matter and leave behind chunks of calcified bone; non-calcified bone is destroyed in this process.
As opposed to a widely held belief, ashes are not formed after this process. Post-incineration, the items left behind may include metal screws, dental implants, dental gold, prosthetic parts, surgical screws, and other non-consumed metal items. These items are separated from the calcified bone using strong magnets. The final dried bone chunks are then pulverized into a finer powder.
A Final Word
Regarding the question of whether bones can really be broken down… the answer is sort of. The organic materials are burnt at lower temperatures, but for the core mineral to break down, extremely high temperatures are required and for extended periods. The amount of time required and the exact temperature will vary depending on factors affecting the porosity and strength of hydroxyapatite.
To reach such high temperatures for extended periods of time is difficult, which prevents the bones from disintegrating in most car crashes and other fire-related accidents. This preserves them for forensic scientists to reveal the truth that only hydroxyapatite can now tell.
References (click to expand)
- Boskey, A. L. (2013, December 4). Bone composition: relationship to bone fragility and antiosteoporotic drug effects. BoneKEy Reports. Portico.
- Hydroxyapatite | Ca5HO13P3 - PubChem. PubChem
- Pokhrel, S. (2018). Hydroxyapatite: Preparation, Properties and Its Biomedical Applications. Advances in Chemical Engineering and Science. Scientific Research Publishing, Inc.
- SINTERING BEHAVIOR AND KINETIC EVALUATION OF HYDROXYAPATITE BIO-CERAMICS FROM BOVINE BONE - www.irsm.cas.cz
- Remotely measuring the temperature of Kīlauea lava. The United States Geological Survey
- Liao, C.-J., Lin, F.-H., Chen, K.-S., & Sun, J.-S. (1999, October). Thermal decomposition and reconstitution of hydroxyapatite in air atmosphere. Biomaterials. Elsevier BV.
- Cihlář, J., Buchal, A., & Trunec, M. (1999). []. Journal of Materials Science. Springer Science and Business Media LLC.
- Ou, S.-F., Chiou, S.-Y., & Ou, K.-L. (2013, May). Phase transformation on hydroxyapatite decomposition. Ceramics International. Elsevier BV.
- How Is A Body Cremated?. cremationresource.org











