Table of Contents (click to expand)
Water is a good conductor of heat, while the fibers in clothes are not. When you spray water on clothes before ironing, the heat from the iron is distributed evenly throughout the fabric, rather than being concentrated in one area. This prevents the fabric from becoming shrivelled or damaged.
It is a warm sunny day, so you decide to dry your washed clothes since the sun is shining. Once they’ve all dried out, it’s time to iron them to perfection. You set your shirt on the ironing board, plug in the iron, fill it up with water and go swoosh-swish-woosh over the shirt. As you see your shirt soaking in the water droplets, you press down with the warm iron plate and slide it over the creases.
 As you do that, you can feel the iron glide smoothly over the damp fabric, letting out a mist of steam and eliminating the roll-overs and wrinkles. However, why are clothes not ironed directly once they’re dry? In reality, moist fabrics are better candidates for being rolled than your regular dry clothes, but why is that the case?
As you do that, you can feel the iron glide smoothly over the damp fabric, letting out a mist of steam and eliminating the roll-overs and wrinkles. However, why are clothes not ironed directly once they’re dry? In reality, moist fabrics are better candidates for being rolled than your regular dry clothes, but why is that the case?
Recommended Video for you:
The Interaction Of Water With Fabric
The answer lies deep in the fabric itself, which is made of millions of fibres that get re-enforced and tangled every time you wash them. Upon drying the clothes, the fibres get more closely packed together again, as they slowly lose their moisture through evaporation in the atmosphere. This leads to the formation of folds and creases. Fibres like cotton and wool are bad conductors of heat. Every time you heat press them, the heat doesn’t spread evenly over the whole area of cloth, which leaves them shrivelled and looking terrible. However, when you spray a mist of water on the fabric first, the water molecules seep, in between the fibres. Water, which is a good conductor of heat, distributes the heat evenly along the whole area of the fabric, thus properly pressing the cloth. The same idea can be applied to steam, which is the vapour form of water. Unlike water, which must be externally heated with the iron, the steam itself is already heated.
When passing steam through the fabric, it evenly distributes the heat throughout the material, which opens up the wrinkles and folds. You’ve probably heard that if your clothes wrinkle while travelling, you can smooth them out by hanging them in the bathroom while you take a hot shower, but have you ever wondered why? Exactly the same reason, we just gave! The steam will loosen the wrinkles, leaving the outfit looking freshly pressed. One more advantage of damp ironing or steam pressing is that it kills odour-causing bacteria and removes allergens that attract dust mites.

Interactions On The Molecular Level
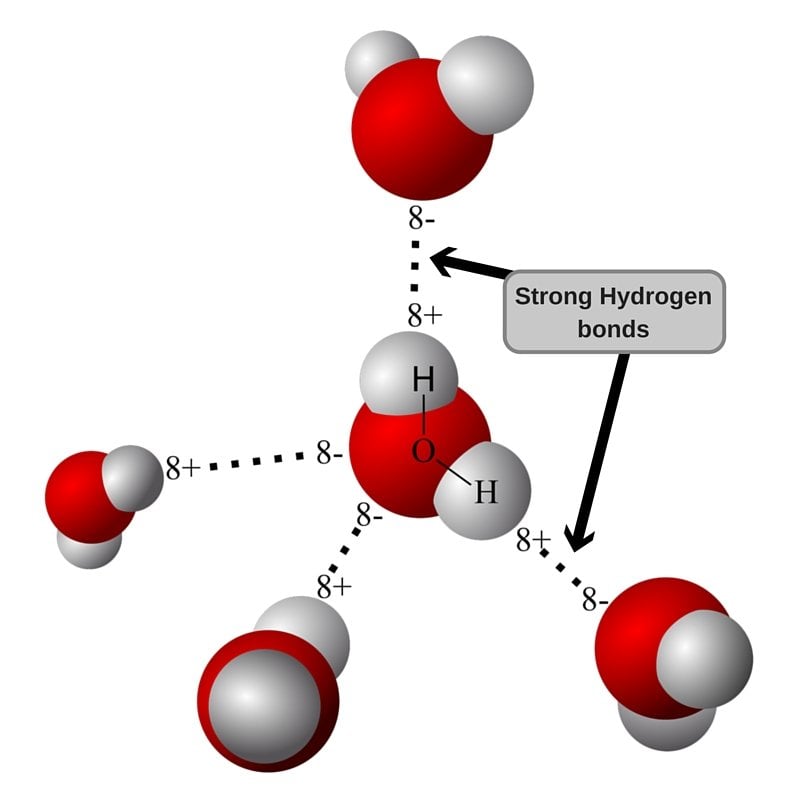
One thing we need to understand is that wrinkles occur at a molecular level. The culmination of these deformities on the whole of the fabric leads to the formation of folds and creases that are visible to our eyes. Molecules are connected to each other through weak forces of attraction known as hydrogen bonds. When you spray water on your shirt, something deeper has taken place, aside from you sulking over your shirt getting wet. Water molecules have led to the weakening of the hydrogen bonds between the fibres, due to the presence of oxygen atoms in water molecules. Once the moisture disappears, the bonds form again. This is why wrinkles form when your laundry is dried in the first place. Ironing merely realigns the fibre molecules, whereas water loosens entangled fibres.
Just a word of caution, not all fabrics are suited for steam ironing. Materials ranging from delicate silks to dry woollens may show brilliant results with steam ironing, owing to their fabric structures, but materials like chiffon and velvet lose their structure upon contact with any form of water, let alone heated steam from an iron. The fibres, on a molecular level, get damaged by the moist heating method. The same goes for leather, suede and waxed materials, which would instantly melt upon exposure to steam.
Conclusion
Voila, we’ve learnt a necessary life hack today! That being said, one thing we need to remember is that not every piece of clothing is made of the same fabric. The ironing method is best suited for cotton clothes, which have a lot of entangled cotton fibres, as compared to rayon and silk fabrics. Therefore, the next time you decide to iron one of your silk shirts or your mom’s silk gowns, carefully check the heat settings on the iron, rather than just dousing the item with water and going wild with the heavy heat treatment!